What is Global Medical Quality Management Systems (QMS) Market?
Global Medical Quality Management Systems (QMS) Market refers to the comprehensive framework and tools used by healthcare organizations worldwide to ensure that their products and services meet the highest standards of quality and safety. These systems are crucial in the medical field as they help in maintaining compliance with regulatory requirements, improving patient outcomes, and reducing risks associated with medical errors. The market encompasses various software and services designed to streamline processes such as document control, training management, audit management, and corrective and preventive actions (CAPA). By implementing a robust QMS, healthcare providers can enhance operational efficiency, ensure regulatory compliance, and ultimately deliver better patient care. The global reach of these systems means they are used by a wide range of healthcare organizations, from small clinics to large hospitals, and across different regions, each with its own set of regulatory standards and requirements. This widespread adoption underscores the critical role that QMS plays in the healthcare industry, driving continuous improvement and fostering a culture of quality and safety.
Cloud Based, On Premises in the Global Medical Quality Management Systems (QMS) Market:
Cloud-based and on-premises solutions are two primary deployment models in the Global Medical Quality Management Systems (QMS) Market, each offering distinct advantages and challenges. Cloud-based QMS solutions are hosted on remote servers and accessed via the internet, providing flexibility and scalability. These solutions are particularly beneficial for organizations looking to reduce upfront costs and IT infrastructure investments. They offer the advantage of automatic updates, ensuring that the system is always up-to-date with the latest features and regulatory requirements. Additionally, cloud-based systems facilitate remote access, enabling healthcare professionals to access critical information from anywhere, which is particularly useful in today's increasingly mobile and remote work environments. On the other hand, on-premises QMS solutions are installed locally on an organization's own servers and managed by their internal IT staff. This model offers greater control over data security and system customization, which can be crucial for organizations with specific regulatory or operational requirements. On-premises solutions may also provide better performance and reliability, as they are not dependent on internet connectivity. However, they typically require a higher initial investment in hardware and software, as well as ongoing maintenance and support costs. Both deployment models have their own set of benefits and limitations, and the choice between them often depends on an organization's specific needs, resources, and strategic goals. For instance, a large hospital with a dedicated IT department might prefer an on-premises solution for its enhanced control and customization capabilities, while a smaller clinic might opt for a cloud-based solution to take advantage of its cost-effectiveness and ease of implementation. Ultimately, the decision between cloud-based and on-premises QMS solutions should be guided by a thorough assessment of the organization's requirements, budget, and long-term objectives.
Pharmaceutical companies, Medical device companies, Others in the Global Medical Quality Management Systems (QMS) Market:
The usage of Global Medical Quality Management Systems (QMS) Market spans across various sectors within the healthcare industry, including pharmaceutical companies, medical device companies, and other healthcare organizations. In pharmaceutical companies, QMS plays a pivotal role in ensuring that all processes, from drug development to manufacturing and distribution, adhere to stringent regulatory standards. These systems help in managing documentation, tracking changes, conducting audits, and implementing corrective actions, thereby ensuring that the final product is safe, effective, and of high quality. By maintaining comprehensive records and facilitating continuous monitoring, QMS helps pharmaceutical companies in achieving compliance with regulatory bodies such as the FDA and EMA, reducing the risk of costly recalls and enhancing patient safety. In medical device companies, QMS is equally critical. These systems help in managing the entire lifecycle of a medical device, from design and development to production and post-market surveillance. By ensuring that all processes are well-documented and compliant with regulatory standards, QMS helps in minimizing risks and ensuring that the devices are safe and effective for patient use. Additionally, QMS facilitates the management of supplier quality, risk assessments, and customer feedback, which are essential for continuous improvement and innovation in the medical device industry. Other healthcare organizations, such as hospitals, clinics, and research institutions, also benefit significantly from implementing QMS. These systems help in streamlining various operational processes, such as patient care, clinical trials, and laboratory management. By ensuring that all activities are conducted in compliance with regulatory standards and best practices, QMS helps in improving overall efficiency, reducing errors, and enhancing patient outcomes. Furthermore, QMS supports the integration of various functions within an organization, promoting a culture of quality and continuous improvement. In summary, the Global Medical Quality Management Systems (QMS) Market is indispensable across different sectors of the healthcare industry, driving compliance, efficiency, and quality in all aspects of healthcare delivery.
Global Medical Quality Management Systems (QMS) Market Outlook:
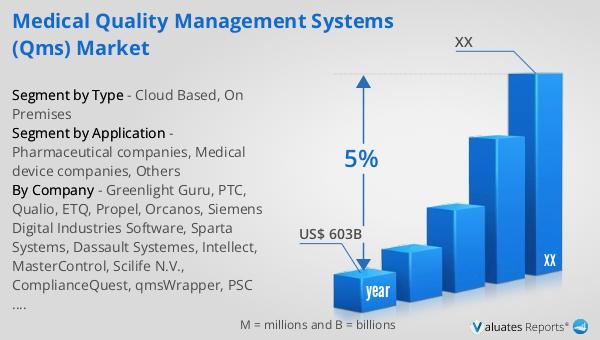
According to our research, the global market for medical devices is projected to reach approximately US$ 603 billion by the year 2023, with an anticipated growth rate of 5% CAGR over the next six years. This significant market size underscores the critical importance of medical devices in the healthcare industry, as well as the growing demand for advanced and innovative solutions. The steady growth rate reflects the continuous advancements in medical technology, increasing prevalence of chronic diseases, and the rising aging population, all of which contribute to the expanding need for medical devices. As the market evolves, there is a heightened focus on ensuring the quality and safety of these devices, which in turn drives the demand for robust Quality Management Systems (QMS). These systems play a crucial role in helping manufacturers comply with stringent regulatory requirements, manage risks, and ensure that their products meet the highest standards of quality and safety. The projected growth of the medical device market highlights the ongoing need for effective QMS solutions that can support the industry's dynamic and complex landscape.
| Report Metric | Details |
| Report Name | Medical Quality Management Systems (QMS) Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Greenlight Guru, PTC, Qualio, ETQ, Propel, Orcanos, Siemens Digital Industries Software, Sparta Systems, Dassault Systemes, Intellect, MasterControl, Scilife N.V., ComplianceQuest, qmsWrapper, PSC Software, CEBOS, CAMA Software, Ideagen Plc, Cority, BatchMaster Software, DHC Business Solutions GmbH, Veeva, AssurX, Sarjen System, WorldAPP, InfinityQS, IQS, Matrix Requirements, Northwest Analytics, PRISYM ID |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
