What is Global Lanreotide Injection Market?
The global Lanreotide Injection market is a specialized segment within the pharmaceutical industry, focusing on the production and distribution of Lanreotide, a synthetic somatostatin analog. Lanreotide is primarily used to treat acromegaly, a hormonal disorder that results from an excess of growth hormone, and certain types of neuroendocrine tumors. The market encompasses various stakeholders, including pharmaceutical companies, healthcare providers, and patients. The demand for Lanreotide injections is driven by the increasing prevalence of these medical conditions, advancements in drug formulations, and the growing awareness about the benefits of early diagnosis and treatment. The market is characterized by rigorous regulatory standards, ongoing research and development activities, and competitive pricing strategies. As a result, companies operating in this market are continually innovating to improve the efficacy, safety, and patient compliance of their products. The global Lanreotide Injection market is poised for steady growth, supported by the rising incidence of target diseases and the expanding healthcare infrastructure in emerging economies.
Sustained Release Formulation, Long-acting Formula in the Global Lanreotide Injection Market:
Sustained release formulations, particularly long-acting formulas, play a crucial role in the global Lanreotide Injection market. These formulations are designed to release the active ingredient, Lanreotide, gradually over an extended period, thereby maintaining therapeutic levels in the bloodstream for longer durations. This approach offers several advantages, including improved patient compliance, reduced dosing frequency, and enhanced therapeutic outcomes. In the context of Lanreotide, long-acting formulations are particularly beneficial for patients with chronic conditions like acromegaly and neuroendocrine tumors, where consistent hormone suppression is essential. The development of these formulations involves sophisticated technologies and rigorous testing to ensure that the drug is released at a controlled rate. One of the key technologies used in sustained release formulations is the use of biodegradable polymers, which encapsulate the drug and degrade slowly over time, releasing the active ingredient in a controlled manner. This not only enhances the drug's efficacy but also minimizes side effects associated with peak drug concentrations. Additionally, long-acting Lanreotide injections reduce the burden on patients, who otherwise would need to adhere to frequent dosing schedules. This is particularly important for patients with chronic conditions, as it improves their quality of life and reduces the likelihood of missed doses. The development and commercialization of sustained release formulations require significant investment in research and development, as well as compliance with stringent regulatory standards. Companies in the Lanreotide Injection market invest heavily in clinical trials to demonstrate the safety and efficacy of their long-acting formulations. These trials are designed to evaluate various parameters, including the pharmacokinetics, pharmacodynamics, and overall clinical outcomes of the drug. The data generated from these trials are then submitted to regulatory authorities for approval. Once approved, these formulations are marketed to healthcare providers and patients, highlighting their benefits over traditional short-acting formulations. The adoption of long-acting Lanreotide injections is also influenced by healthcare policies and reimbursement frameworks, which vary across different regions. In some countries, the cost of long-acting formulations may be covered by insurance, making them more accessible to patients. In other regions, out-of-pocket expenses may limit their adoption. Despite these challenges, the demand for sustained release formulations is expected to grow, driven by the increasing prevalence of target diseases and the ongoing advancements in drug delivery technologies. In summary, sustained release formulations, particularly long-acting Lanreotide injections, represent a significant advancement in the treatment of chronic conditions like acromegaly and neuroendocrine tumors. These formulations offer numerous benefits, including improved patient compliance, reduced dosing frequency, and enhanced therapeutic outcomes. The development and commercialization of these formulations require substantial investment in research and development, as well as compliance with stringent regulatory standards. As the global Lanreotide Injection market continues to evolve, sustained release formulations are expected to play an increasingly important role in meeting the needs of patients and healthcare providers.
Hospital, Clinic, Others in the Global Lanreotide Injection Market:
The usage of Lanreotide injections spans various healthcare settings, including hospitals, clinics, and other medical facilities. In hospitals, Lanreotide injections are often administered to patients with severe or advanced stages of acromegaly and neuroendocrine tumors. Hospitals are equipped with the necessary infrastructure and medical expertise to manage these complex conditions, making them a primary setting for the administration of Lanreotide. In addition to treating inpatients, hospitals also provide outpatient services where patients can receive their injections and undergo regular monitoring. The availability of specialized endocrinologists and oncologists in hospitals ensures that patients receive comprehensive care, including diagnosis, treatment, and follow-up. Clinics, on the other hand, offer a more accessible and convenient option for patients requiring Lanreotide injections. These smaller healthcare facilities are often located in community settings, making it easier for patients to receive their treatments without the need to travel long distances. Clinics typically provide a range of services, including initial consultations, diagnostic tests, and ongoing treatment management. The administration of Lanreotide injections in clinics is usually performed by trained healthcare professionals, such as nurses or general practitioners, under the supervision of a specialist. This approach allows for timely and efficient treatment, reducing the burden on hospital resources and improving patient access to care. Other medical facilities, such as specialized treatment centers and home healthcare services, also play a role in the administration of Lanreotide injections. Specialized treatment centers focus on specific medical conditions, offering targeted therapies and advanced treatment options. These centers often collaborate with hospitals and clinics to provide a continuum of care for patients with chronic conditions. Home healthcare services, on the other hand, offer a more personalized approach, allowing patients to receive their injections in the comfort of their own homes. This option is particularly beneficial for patients with mobility issues or those who require long-term treatment. Home healthcare services are typically provided by trained nurses or healthcare aides, who ensure that the injections are administered safely and effectively. The usage of Lanreotide injections in these various settings highlights the importance of a multidisciplinary approach to patient care. By offering multiple options for treatment administration, healthcare providers can tailor their services to meet the unique needs of each patient. This flexibility is crucial for managing chronic conditions like acromegaly and neuroendocrine tumors, where consistent and timely treatment is essential for optimal outcomes. In summary, the usage of Lanreotide injections spans a range of healthcare settings, including hospitals, clinics, and other medical facilities. Each setting offers unique advantages, from the comprehensive care provided in hospitals to the accessibility and convenience of clinics and home healthcare services. By leveraging these various options, healthcare providers can ensure that patients receive the best possible care, improving their quality of life and treatment outcomes.
Global Lanreotide Injection Market Outlook:
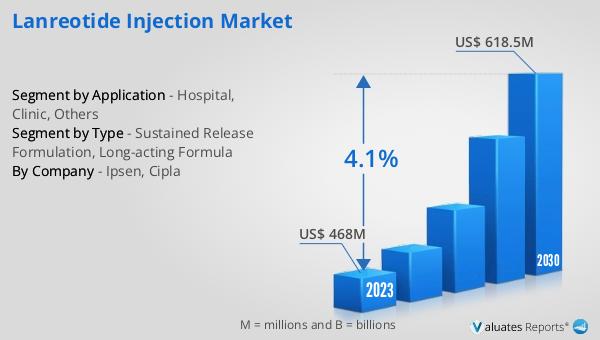
The global Lanreotide Injection market was valued at $468 million in 2023 and is projected to reach $618.5 million by 2030, reflecting a compound annual growth rate (CAGR) of 4.1% during the forecast period from 2024 to 2030. This growth is driven by several factors, including the increasing prevalence of conditions like acromegaly and neuroendocrine tumors, advancements in drug formulations, and the expanding healthcare infrastructure in emerging economies. The market is characterized by intense competition among pharmaceutical companies, each striving to develop more effective and patient-friendly formulations. Regulatory standards play a significant role in shaping the market dynamics, as companies must comply with stringent guidelines to gain approval for their products. The ongoing research and development activities in this field are focused on improving the efficacy, safety, and patient compliance of Lanreotide injections. As a result, the market is witnessing the introduction of innovative long-acting formulations that offer numerous benefits over traditional short-acting versions. These advancements are expected to drive the adoption of Lanreotide injections across various healthcare settings, including hospitals, clinics, and home healthcare services. In summary, the global Lanreotide Injection market is poised for steady growth, supported by the rising incidence of target diseases and the continuous advancements in drug delivery technologies.
| Report Metric | Details |
| Report Name | Lanreotide Injection Market |
| Accounted market size in 2023 | US$ 468 million |
| Forecasted market size in 2030 | US$ 618.5 million |
| CAGR | 4.1% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Ipsen, Cipla |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
