What is Global Divalproex Sodium API Market?
The Global Divalproex Sodium API Market refers to the worldwide market for the active pharmaceutical ingredient (API) used in the production of Divalproex Sodium, a medication primarily prescribed for the treatment of epilepsy, bipolar disorder, and to prevent migraine headaches. This market encompasses the production, distribution, and sale of Divalproex Sodium API to pharmaceutical companies that manufacture the final dosage forms, such as tablets and capsules. The demand for Divalproex Sodium API is driven by the prevalence of neurological and psychiatric disorders, increasing awareness about mental health, and the growing need for effective treatment options. The market is characterized by the presence of several key players who compete based on product quality, purity levels, and pricing. Additionally, regulatory standards and approvals play a crucial role in shaping the market dynamics, as compliance with stringent quality and safety guidelines is essential for market entry and sustainability. The global reach of this market highlights its significance in addressing critical health issues across different regions, making it a vital component of the pharmaceutical industry.
Purity≥98%, Purity<98% in the Global Divalproex Sodium API Market:
In the Global Divalproex Sodium API Market, the purity of the API is a critical factor that influences its efficacy, safety, and overall quality. Purity levels are typically categorized into two main segments: Purity≥98% and Purity<98%. APIs with a purity of 98% or higher are considered to be of superior quality, offering enhanced therapeutic benefits and reduced risk of side effects. These high-purity APIs are preferred by pharmaceutical companies for the production of medications that require stringent quality standards, such as those used in the treatment of severe neurological and psychiatric conditions. The manufacturing process for high-purity APIs involves advanced techniques and rigorous quality control measures to ensure that impurities are minimized and the final product meets the required specifications. On the other hand, APIs with a purity of less than 98% may still be effective but are generally considered to be of lower quality. These APIs may contain higher levels of impurities, which can affect their stability, efficacy, and safety profile. Pharmaceutical companies that use lower-purity APIs may do so to reduce production costs, but this can potentially compromise the quality of the final product. The choice between high-purity and lower-purity APIs depends on various factors, including the intended use of the medication, regulatory requirements, and cost considerations. In the context of the Global Divalproex Sodium API Market, the demand for high-purity APIs is expected to remain strong due to the increasing emphasis on quality and safety in the pharmaceutical industry. However, there is also a market for lower-purity APIs, particularly in regions with less stringent regulatory standards or where cost constraints are a significant concern. Overall, the purity of Divalproex Sodium API is a key determinant of its market value and plays a crucial role in shaping the competitive landscape of the industry.
Divalproex Sodium Tablets, Others in the Global Divalproex Sodium API Market:
The Global Divalproex Sodium API Market finds its primary application in the production of Divalproex Sodium Tablets, which are widely used for the treatment of epilepsy, bipolar disorder, and migraine prevention. These tablets are formulated to deliver the active ingredient in a controlled manner, ensuring optimal therapeutic effects while minimizing potential side effects. The efficacy of Divalproex Sodium Tablets is largely dependent on the quality and purity of the API used in their production. High-purity APIs contribute to the stability and effectiveness of the tablets, making them a preferred choice for healthcare providers and patients. In addition to tablets, Divalproex Sodium API is also used in other dosage forms, such as extended-release capsules and oral solutions, which offer alternative administration options for patients with different needs and preferences. These alternative dosage forms are particularly beneficial for patients who have difficulty swallowing tablets or require a more gradual release of the medication into their system. The versatility of Divalproex Sodium API in various dosage forms underscores its importance in the pharmaceutical industry and its role in addressing diverse patient needs. Furthermore, the use of Divalproex Sodium API extends beyond human medicine, as it is also employed in veterinary applications for the treatment of similar neurological conditions in animals. This broad range of applications highlights the significance of Divalproex Sodium API in both human and veterinary healthcare, making it a valuable asset in the global pharmaceutical market. The continuous research and development efforts aimed at improving the formulation and delivery of Divalproex Sodium-based medications further contribute to the growth and evolution of the market. As new therapeutic indications and advanced delivery systems are explored, the demand for high-quality Divalproex Sodium API is expected to increase, reinforcing its critical role in the treatment of neurological and psychiatric disorders.
Global Divalproex Sodium API Market Outlook:
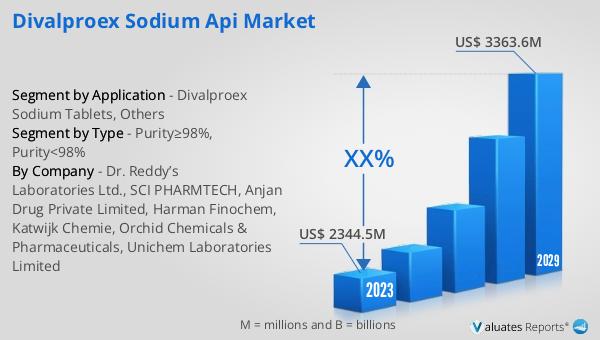
The global Divalproex Sodium API market was valued at US$ 2188.5 million in 2023 and is anticipated to reach US$ 3363.6 million by 2030, witnessing a CAGR of 6.2% during the forecast period from 2024 to 2030. North America holds the largest market share, accounting for approximately 41% of the total market. Europe follows, with about 26% of the market share. The top three companies in the market collectively occupy around 49% of the market share. This market outlook indicates a robust growth trajectory driven by the increasing prevalence of neurological and psychiatric disorders, coupled with the rising demand for effective treatment options. The significant market share held by North America and Europe underscores the advanced healthcare infrastructure and high awareness levels in these regions. The dominance of the top three companies highlights the competitive nature of the market, where leading players leverage their expertise, extensive product portfolios, and strong distribution networks to maintain their market position. As the market continues to evolve, the focus on quality, regulatory compliance, and innovative product development will remain key factors influencing the competitive landscape and overall growth of the Global Divalproex Sodium API Market.
| Report Metric | Details |
| Report Name | Divalproex Sodium API Market |
| Accounted market size in 2023 | US$ 2188.5 million |
| Forecasted market size in 2030 | US$ 3363.6 million |
| CAGR | 6.2% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Dr. Reddy’s Laboratories Ltd., SCI PHARMTECH, Anjan Drug Private Limited, Harman Finochem, Katwijk Chemie, Orchid Chemicals & Pharmaceuticals, Unichem Laboratories Limited |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |