What is Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) Market?
The Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) Market is a specialized segment within the pharmaceutical industry, focusing on the production and distribution of a high-purity form of Eicosapentaenoic Acid. This particular type of EPA is utilized in various medical formulations and treatments, owing to its significant health benefits, especially in cardiovascular health. Eicosapentaenoic Acid, a crucial omega-3 fatty acid primarily found in fish oil, plays a vital role in reducing inflammation and blood clotting. In the pharmaceutical grade, its purity and quality are paramount to ensure its efficacy and safety for medical use. The market for this high-grade EPA has seen substantial growth due to the increasing awareness of its health benefits, coupled with the rising prevalence of heart-related diseases globally. As a result, pharmaceutical companies are investing heavily in research and development to produce EPA with the highest purity levels, meeting stringent regulatory standards for therapeutic use. This market's expansion is also fueled by the growing demand for omega-3 supplements among health-conscious consumers, further driving the need for high-quality, pharmaceutical-grade Eicosapentaenoic Acid.
Purity 96.5%, Purity 99% in the Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) Market:
In the realm of the Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) Market, the distinction between Purity 96.5% and Purity 99% EPA is of paramount importance, as it directly impacts the compound's efficacy and potential applications in healthcare. Eicosapentaenoic Acid, an omega-3 fatty acid, is critical for maintaining cardiovascular health and preventing diseases. The 96.5% purity EPA is considered high-quality and is suitable for most pharmaceutical applications, including the formulation of cardiovascular and hypolipidemic drugs. It is effective in reducing triglyceride levels, lowering the risk of heart attacks, and managing blood pressure. On the other hand, the 99% purity EPA represents the pinnacle of refinement, offering unparalleled purity that is essential for specific, sensitive medical applications. This ultra-pure form is often used in advanced therapeutic products, where even minor impurities can affect patient outcomes. The production process for achieving such high purity levels is complex and costly, involving sophisticated technology and stringent quality control measures. As a result, 99% purity EPA commands a premium in the market, reflecting its superior quality and effectiveness. The demand for both purity levels is driven by the growing body of research underscoring the benefits of EPA in treating a wide range of health conditions, from mental health disorders to inflammatory diseases. Pharmaceutical companies are continuously exploring new ways to harness the potential of EPA, tailoring their products to meet the evolving needs of healthcare providers and patients alike. The distinction between these purity levels highlights the market's dynamic nature and the ongoing quest for products that can deliver the best health outcomes.
Cardiovascular Disease Drugs, Hypolipidemic Drugs in the Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) Market:
The usage of Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) in the areas of Cardiovascular Disease Drugs and Hypolipidemic Drugs is a testament to its critical role in modern medicine. In the treatment of cardiovascular diseases, EPA is utilized for its potent anti-inflammatory and anti-thrombotic properties, which help in reducing the risk of heart attacks and strokes. It works by inhibiting the formation of blood clots and improving lipid profiles, which is crucial for patients suffering from cardiovascular conditions. The high purity of pharmaceutical-grade EPA ensures that it can be effectively used in the formulation of drugs aimed at treating these diseases, offering a safer alternative to traditional medications that may have adverse side effects. Similarly, in the development of hypolipidemic drugs, which are designed to lower lipid levels in the blood, EPA plays a vital role. Its ability to significantly reduce triglyceride levels makes it an invaluable component of treatments aimed at preventing hyperlipidemia, a condition that can lead to severe cardiovascular issues if left untreated. The pharmaceutical-grade EPA, with its high purity, is particularly suited for these applications, ensuring that patients receive the maximum therapeutic benefits with minimal risks. The ongoing research and clinical trials focusing on EPA's efficacy further underscore its importance in the pharmaceutical industry, driving the demand for high-quality, pharmaceutical-grade EPA in the development of innovative drugs for cardiovascular and lipid-related disorders.
Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) Market Outlook:
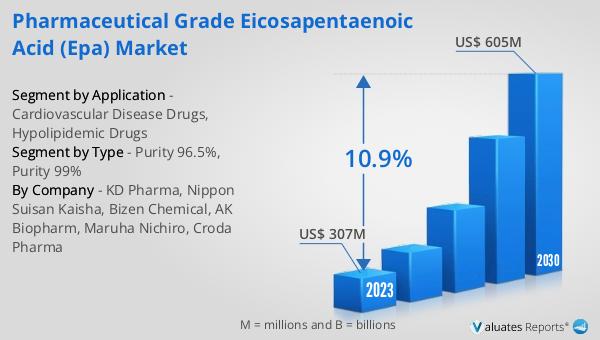
The market outlook for Global Pharmaceutical Grade Eicosapentaenoic Acid (EPA) presents a promising future, with the industry's valuation set at US$ 307 million in 2023 and projected to almost double to US$ 605 million by 2030. This impressive growth trajectory, marked by a Compound Annual Growth Rate (CAGR) of 10.9% during the forecast period from 2024 to 2030, underscores the increasing recognition of EPA's therapeutic benefits and its expanding application in the pharmaceutical sector. The surge in demand for high-quality, pharmaceutical-grade EPA is driven by its critical role in developing treatments for cardiovascular diseases and conditions requiring lipid level management. As awareness of the health benefits associated with omega-3 fatty acids continues to grow among healthcare professionals and consumers alike, the market for pharmaceutical-grade EPA is expected to witness substantial expansion. This growth is further supported by ongoing research and development activities aimed at exploring new therapeutic uses for EPA, reinforcing its position as a key ingredient in the pharmaceutical industry's quest to improve patient outcomes. The forecasted growth reflects the market's potential to contribute significantly to the advancement of healthcare, offering promising prospects for both manufacturers and consumers of pharmaceutical-grade Eicosapentaenoic Acid.
| Report Metric | Details |
| Report Name | Pharmaceutical Grade Eicosapentaenoic Acid (EPA) Market |
| Accounted market size in 2023 | US$ 307 million |
| Forecasted market size in 2030 | US$ 605 million |
| CAGR | 10.9% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | KD Pharma, Nippon Suisan Kaisha, Bizen Chemical, AK Biopharm, Maruha Nichiro, Croda Pharma |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |