What is Global EDC (Electronic Data Capture) System Market?
The Global EDC (Electronic Data Capture) System Market refers to the worldwide industry focused on the development, deployment, and utilization of electronic systems designed to capture and manage data electronically. These systems are primarily used in clinical trials and research to collect, store, and analyze data efficiently and accurately. EDC systems replace traditional paper-based methods, offering a more streamlined and error-free approach to data management. They are essential in ensuring data integrity, compliance with regulatory standards, and facilitating faster decision-making processes. The market encompasses various types of EDC systems, including web-based and cloud-based solutions, which cater to the diverse needs of pharmaceutical companies, biotech organizations, medical device manufacturers, academic researchers, and other stakeholders involved in clinical research and trials. The adoption of EDC systems is driven by the increasing complexity of clinical trials, the need for real-time data access, and the growing emphasis on data accuracy and regulatory compliance. As the demand for efficient data management solutions continues to rise, the Global EDC System Market is expected to expand, offering innovative technologies and services to meet the evolving needs of the industry.
Web-based, Cloud-based in the Global EDC (Electronic Data Capture) System Market:
Web-based and cloud-based EDC (Electronic Data Capture) systems are two prominent types of solutions within the Global EDC System Market, each offering unique advantages and functionalities. Web-based EDC systems are accessed through a web browser, allowing users to enter, manage, and analyze data from any location with internet access. These systems are typically hosted on a central server, which can be managed by the organization or a third-party service provider. The primary benefit of web-based EDC systems is their accessibility, as they enable researchers and clinical trial managers to collaborate and share data in real-time, regardless of their geographical location. This feature is particularly valuable in multi-center clinical trials, where data needs to be collected and analyzed from various sites. Additionally, web-based EDC systems often come with user-friendly interfaces and customizable features, making them adaptable to the specific needs of different research projects. On the other hand, cloud-based EDC systems take the concept of web-based solutions a step further by leveraging cloud computing technology. These systems are hosted on cloud servers, which offer enhanced scalability, flexibility, and cost-effectiveness. Cloud-based EDC systems provide users with the ability to scale their data storage and processing capabilities according to their needs, without the need for significant upfront investments in hardware and infrastructure. This scalability is particularly beneficial for organizations conducting large-scale clinical trials or those experiencing rapid growth. Furthermore, cloud-based EDC systems often come with advanced security features, such as data encryption and multi-factor authentication, ensuring that sensitive clinical data is protected against unauthorized access and breaches. Both web-based and cloud-based EDC systems offer significant advantages over traditional paper-based methods, including improved data accuracy, reduced risk of errors, and faster data processing times. These systems also facilitate compliance with regulatory requirements, as they often include built-in audit trails and validation checks to ensure data integrity. Moreover, the use of electronic systems streamlines the data collection process, reducing the administrative burden on researchers and allowing them to focus more on the scientific aspects of their work. As the Global EDC System Market continues to evolve, the adoption of web-based and cloud-based solutions is expected to increase, driven by the need for efficient, reliable, and secure data management tools in clinical research and trials.
Pharma & Biotech Organizations, Medical Device, Academic Reserch, Other in the Global EDC (Electronic Data Capture) System Market:
The usage of Global EDC (Electronic Data Capture) System Market spans across various sectors, including Pharma & Biotech Organizations, Medical Device companies, Academic Research, and other areas. In Pharma & Biotech Organizations, EDC systems are crucial for managing the vast amounts of data generated during clinical trials. These systems enable researchers to collect, store, and analyze data in real-time, ensuring that the information is accurate and up-to-date. This real-time data access allows for quicker decision-making and more efficient trial management, ultimately speeding up the drug development process. Additionally, EDC systems help pharmaceutical companies comply with regulatory requirements by providing a secure and auditable record of all data collected during the trial. In the Medical Device sector, EDC systems play a similar role in managing data from clinical trials and post-market surveillance studies. These systems help manufacturers collect and analyze data on the safety and efficacy of their devices, ensuring that they meet regulatory standards and can be safely used by patients. The ability to quickly and accurately collect data also allows for faster identification of any potential issues with the device, enabling manufacturers to address these problems promptly and maintain compliance with regulatory requirements. Academic Research institutions also benefit from the use of EDC systems, as they enable researchers to manage and analyze data from various studies more efficiently. These systems provide a centralized platform for data collection, storage, and analysis, making it easier for researchers to collaborate and share information. This streamlined approach to data management helps to improve the overall quality and reliability of research findings, ultimately contributing to the advancement of scientific knowledge. Other areas where EDC systems are used include contract research organizations (CROs), government agencies, and non-profit organizations involved in clinical research. CROs, for example, use EDC systems to manage data from multiple clinical trials conducted on behalf of their clients. These systems help CROs ensure that data is collected and analyzed consistently across all trials, improving the overall quality and reliability of the results. Government agencies and non-profit organizations also use EDC systems to manage data from public health studies and other research initiatives, ensuring that the information collected is accurate and can be used to inform policy decisions and improve public health outcomes.
Global EDC (Electronic Data Capture) System Market Outlook:
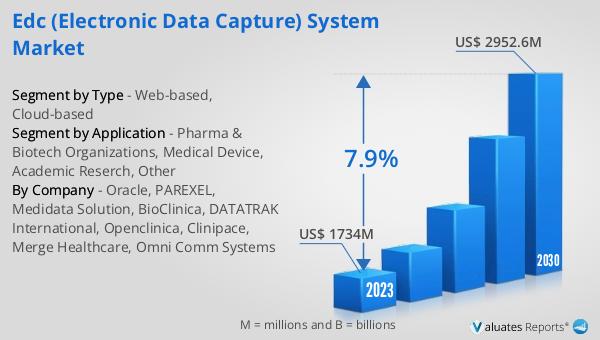
The global EDC (Electronic Data Capture) System market was valued at US$ 1734 million in 2023 and is anticipated to reach US$ 2952.6 million by 2030, witnessing a CAGR of 7.9% during the forecast period 2024-2030. This significant growth reflects the increasing demand for efficient and reliable data management solutions in clinical research and trials. The adoption of EDC systems is driven by the need for real-time data access, improved data accuracy, and compliance with regulatory standards. As the complexity of clinical trials continues to rise, the demand for advanced EDC systems is expected to grow, offering innovative technologies and services to meet the evolving needs of the industry. The market encompasses various types of EDC systems, including web-based and cloud-based solutions, which cater to the diverse needs of pharmaceutical companies, biotech organizations, medical device manufacturers, academic researchers, and other stakeholders involved in clinical research and trials. The expansion of the Global EDC System Market is a testament to the growing importance of efficient data management in the healthcare and research sectors.
| Report Metric | Details |
| Report Name | EDC (Electronic Data Capture) System Market |
| Accounted market size in 2023 | US$ 1734 million |
| Forecasted market size in 2030 | US$ 2952.6 million |
| CAGR | 7.9% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Oracle, PAREXEL, Medidata Solution, BioClinica, DATATRAK International, Openclinica, Clinipace, Merge Healthcare, Omni Comm Systems |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
