What is Global Cell and Gene Therapy CDMO Services Market?
The Global Cell and Gene Therapy CDMO (Contract Development and Manufacturing Organization) Services Market is a rapidly expanding sector within the pharmaceutical industry, focusing on providing specialized manufacturing and development services for cell and gene therapies. These therapies represent a new frontier in medicine, offering potential cures and treatments for a wide range of diseases, including various cancers, genetic disorders, and autoimmune diseases. The market's growth is driven by the increasing demand for these advanced therapies, which require complex production processes, stringent regulatory compliance, and specialized knowledge. As a result, biotechnology and pharmaceutical companies are increasingly partnering with CDMOs that have the expertise and facilities to develop, manufacture, and package cell and gene therapies. This collaboration allows therapy developers to accelerate the path from research to market, ensuring that these groundbreaking treatments can reach patients more quickly. With a significant compound annual growth rate (CAGR) projected, the market is set to expand substantially, reflecting the growing importance of cell and gene therapies in modern healthcare.
Autologous Cell Therapy Products, Allogeneic Cell Therapy Products in the Global Cell and Gene Therapy CDMO Services Market:
Autologous and Allogeneic Cell Therapy Products are pivotal components in the Global Cell and Gene Therapy CDMO Services Market, each with unique characteristics and applications. Autologous cell therapy products are derived from a patient's own cells, which are collected, possibly modified, and then reintroduced into the patient's body to treat or manage diseases. This personalized approach minimizes the risk of immune rejection and offers targeted treatment options for conditions such as certain cancers, blood disorders, and wounds. On the other hand, Allogeneic cell therapy products are manufactured from donor cells, not the recipient's own cells. These products offer the advantage of being readily available and can be produced in larger batches, making them potentially more cost-effective and accessible. They are used in a range of treatments, including regenerative medicine and immune system modulation. However, the risk of immune rejection is higher with allogeneic therapies, necessitating careful matching and sometimes the use of immunosuppressive drugs. The Global Cell and Gene Therapy CDMO Services Market plays a crucial role in the development and manufacturing of both autologous and allogeneic therapies. CDMOs provide the expertise, technology, and infrastructure necessary to navigate the complex production processes, regulatory requirements, and quality control standards essential for bringing these innovative therapies to market. As the demand for cell and gene therapies grows, the importance of CDMOs in ensuring the efficient, safe, and effective production of autologous and allogeneic cell therapy products becomes increasingly evident.
SMBs, Large Companies in the Global Cell and Gene Therapy CDMO Services Market:
The Global Cell and Gene Therapy CDMO Services Market plays a significant role in supporting both Small and Medium-sized Businesses (SMBs) and Large Companies in the development and manufacturing of cell and gene therapies. For SMBs, which often lack the extensive resources and infrastructure of larger companies, CDMOs provide essential services that enable these smaller entities to advance their innovative therapies through the development pipeline. This includes access to specialized expertise, state-of-the-art facilities, and regulatory guidance, which can be prohibitively expensive for SMBs to develop in-house. As a result, partnering with CDMOs allows these businesses to focus on their core competencies, such as research and development, while leveraging the CDMO's manufacturing capabilities to bring therapies to market more efficiently. For Large Companies, CDMOs offer scalability and flexibility in manufacturing. These companies may have their own production facilities but turn to CDMOs to manage overflow, access specialized technologies, or expedite the development of certain products. By collaborating with CDMOs, large companies can optimize their production processes, reduce time to market, and manage costs more effectively, all while maintaining high standards of quality and compliance. Thus, the Global Cell and Gene Therapy CDMO Services Market is instrumental in facilitating the growth and success of both SMBs and large companies within the rapidly evolving cell and gene therapy sector.
Global Cell and Gene Therapy CDMO Services Market Outlook:
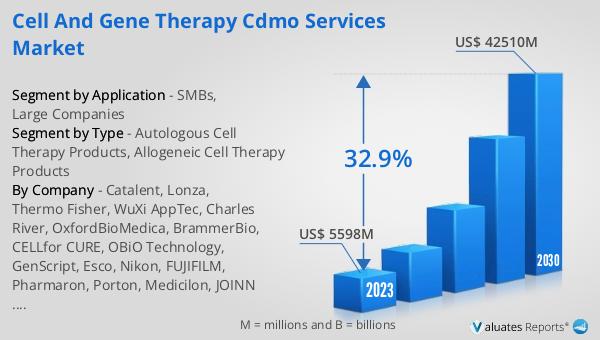
The market outlook for the Global Cell and Gene Therapy CDMO Services Market is exceptionally promising, with the sector's value estimated at US$ 5598 million in 2023, and projections suggest it will soar to US$ 42510 million by 2030. This remarkable growth trajectory, characterized by a compound annual growth rate (CAGR) of 32.9% from 2024 to 2030, underscores the burgeoning demand for cell and gene therapy development and manufacturing services. The surge in market value reflects the increasing investment in cell and gene therapies, driven by their potential to offer groundbreaking treatments for a wide array of diseases. As these therapies move from research to clinical trials and onto the market, the need for specialized CDMO services that can navigate the complex production and regulatory landscapes is more critical than ever. This growth is not just a testament to the advances in medical science but also to the vital role that CDMOs play in bringing these innovative treatments from the laboratory to the patients who need them. The market's expansion is indicative of the broader trends in healthcare towards more personalized and effective treatment options, highlighting the importance of the cell and gene therapy sector in the future of medicine.
| Report Metric | Details |
| Report Name | Cell and Gene Therapy CDMO Services Market |
| Accounted market size in 2023 | US$ 5598 in million |
| Forecasted market size in 2030 | US$ 42510 million |
| CAGR | 32.9% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Catalent, Lonza, Thermo Fisher, WuXi AppTec, Charles River, OxfordBioMedica, BrammerBio, CELLfor CURE, OBiO Technology, GenScript, Esco, Nikon, FUJIFILM, Pharmaron, Porton, Medicilon, JOINN Laboratories, Sofpromed, eXmoor, OmniaBio, RoslinCT, BioCentriq, PolTREG, Miltenyi Bioindustry |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
