What is Global Regulatory Document Management Software (rDMS) Market?
Global Regulatory Document Management Software (rDMS) Market is a specialized segment within the broader software industry that focuses on managing and organizing regulatory documents. These documents are crucial for companies that operate in highly regulated industries, such as pharmaceuticals, biotechnology, medical devices, and chemicals. The software helps organizations streamline their document management processes, ensuring compliance with various regulatory standards and guidelines. By using rDMS, companies can efficiently store, retrieve, and manage documents, reducing the risk of non-compliance and potential legal issues. The market for rDMS is driven by the increasing complexity of regulatory requirements across different industries and regions. As companies expand globally, they face the challenge of adhering to diverse regulatory frameworks, making rDMS an essential tool for maintaining compliance. Additionally, the growing emphasis on data security and the need for efficient document management solutions further contribute to the demand for rDMS. Overall, the Global Regulatory Document Management Software Market plays a vital role in helping organizations navigate the complex regulatory landscape, ensuring that they remain compliant and competitive in their respective industries.
Cloud Based, On-Premises in the Global Regulatory Document Management Software (rDMS) Market:
In the Global Regulatory Document Management Software (rDMS) Market, there are two primary deployment models: cloud-based and on-premises. Each model offers distinct advantages and challenges, catering to different organizational needs and preferences. Cloud-based rDMS solutions are hosted on remote servers and accessed via the internet. This model offers several benefits, including scalability, flexibility, and cost-effectiveness. Organizations can easily scale their storage and processing capabilities as their needs grow, without the need for significant upfront investments in hardware. Additionally, cloud-based solutions enable remote access, allowing employees to access documents from anywhere, which is particularly beneficial for companies with a distributed workforce. The cloud model also reduces the burden on internal IT teams, as the service provider handles maintenance, updates, and security. However, some organizations may have concerns about data security and privacy, as sensitive regulatory documents are stored off-site. On the other hand, on-premises rDMS solutions are installed and operated on the organization's own servers. This model provides greater control over data security and compliance, as companies can implement their own security measures and protocols. On-premises solutions are often preferred by organizations with stringent data protection requirements or those operating in regions with strict data sovereignty laws. However, this model requires significant upfront investment in hardware and infrastructure, as well as ongoing maintenance and support from internal IT teams. Additionally, on-premises solutions may lack the flexibility and scalability of cloud-based models, making it challenging for organizations to adapt to changing needs. Despite these challenges, some companies prefer the on-premises model for its perceived security advantages and control over data. Ultimately, the choice between cloud-based and on-premises rDMS solutions depends on various factors, including the organization's size, industry, regulatory requirements, and IT capabilities. Companies must carefully evaluate their needs and priorities to determine the most suitable deployment model for their regulatory document management needs. As the rDMS market continues to evolve, we may see further innovations and hybrid models that combine the best features of both cloud-based and on-premises solutions, offering organizations even greater flexibility and control.
Pharmaceuticals and Biotechnology, Medical Device Industry, Chemistry and Chemical Industry, Food and Beverage Industry, Energy and Utilities, Other in the Global Regulatory Document Management Software (rDMS) Market:
The Global Regulatory Document Management Software (rDMS) Market finds extensive usage across various industries, each with unique regulatory requirements and challenges. In the pharmaceuticals and biotechnology sectors, rDMS is crucial for managing the vast array of documents required for drug development, clinical trials, and regulatory submissions. These industries are subject to stringent regulations from bodies like the FDA and EMA, making efficient document management essential for compliance and successful product approvals. rDMS helps streamline the documentation process, ensuring that all necessary documents are organized, accessible, and up-to-date. In the medical device industry, rDMS plays a similar role, helping companies manage documents related to product design, testing, and regulatory submissions. With the increasing complexity of medical devices and the growing emphasis on patient safety, effective document management is critical for ensuring compliance with regulatory standards and facilitating market entry. The chemistry and chemical industry also relies on rDMS to manage documents related to product formulations, safety data sheets, and regulatory compliance. These industries face a complex web of regulations governing the production, handling, and disposal of chemicals, making efficient document management essential for maintaining compliance and minimizing risks. In the food and beverage industry, rDMS is used to manage documents related to food safety, quality control, and regulatory compliance. With increasing consumer awareness and regulatory scrutiny, companies must ensure that their products meet stringent safety and quality standards. rDMS helps streamline the documentation process, ensuring that all necessary documents are organized, accessible, and up-to-date. The energy and utilities sector also benefits from rDMS, as companies must comply with a wide range of regulations related to environmental protection, safety, and operational efficiency. rDMS helps manage documents related to permits, inspections, and compliance reporting, ensuring that companies meet regulatory requirements and avoid potential fines or penalties. Finally, other industries, such as finance, healthcare, and manufacturing, also utilize rDMS to manage regulatory documents and ensure compliance with industry-specific regulations. Overall, the Global Regulatory Document Management Software Market plays a vital role in helping organizations across various industries navigate the complex regulatory landscape, ensuring that they remain compliant and competitive.
Global Regulatory Document Management Software (rDMS) Market Outlook:
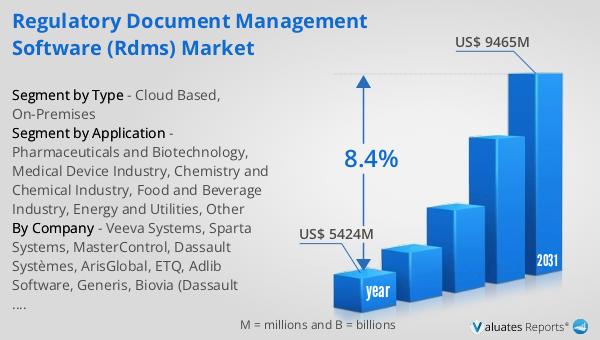
The global market for Regulatory Document Management Software (rDMS) was valued at $5,424 million in 2024 and is anticipated to grow significantly, reaching an estimated size of $9,465 million by 2031. This growth is expected to occur at a compound annual growth rate (CAGR) of 8.4% during the forecast period. This upward trajectory underscores the increasing importance of rDMS solutions in helping organizations manage their regulatory documents efficiently. As industries become more regulated and the volume of documentation grows, the demand for robust document management solutions is expected to rise. Companies are increasingly recognizing the need for efficient systems to handle the complexities of regulatory compliance, driving the adoption of rDMS across various sectors. The projected growth of the rDMS market reflects the broader trend towards digital transformation and the increasing reliance on technology to streamline business processes. As organizations continue to expand globally and face diverse regulatory requirements, the need for effective document management solutions will only become more critical. The anticipated growth of the rDMS market highlights the vital role these solutions play in helping companies navigate the complex regulatory landscape, ensuring compliance and maintaining a competitive edge.
| Report Metric | Details |
| Report Name | Regulatory Document Management Software (rDMS) Market |
| Accounted market size in year | US$ 5424 million |
| Forecasted market size in 2031 | US$ 9465 million |
| CAGR | 8.4% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Veeva Systems, Sparta Systems, MasterControl, Dassault Systèmes, ArisGlobal, ETQ, Adlib Software, Generis, Biovia (Dassault Systèmes), Amplexor |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
