What is Global Medical Masks Synthetic Blood Penetration Tester Market?
The Global Medical Masks Synthetic Blood Penetration Tester Market is a specialized segment within the broader medical device industry. This market focuses on devices designed to test the resistance of medical masks to synthetic blood penetration, ensuring their effectiveness in protecting healthcare workers and patients from blood-borne pathogens. These testers are crucial in maintaining safety standards and compliance with international regulations, especially in the wake of global health crises like the COVID-19 pandemic. The market encompasses a range of products, from fully automated systems to semi-automatic devices, catering to different needs and budgets of healthcare facilities and mask manufacturers. As the demand for high-quality medical masks continues to rise, driven by increased awareness of infection control and stringent safety standards, the market for these testing devices is expected to grow. This growth is further supported by advancements in technology, which enhance the accuracy and efficiency of these testers, making them indispensable tools in the quality assurance processes of mask production. The market's expansion is also fueled by the increasing adoption of these devices in various regions, reflecting a global commitment to improving healthcare safety and standards.
Fully Automatic Medical Masks Synthetic Blood Penetration Tester, Semi-automatic Medical Masks Synthetic Blood Penetration Tester in the Global Medical Masks Synthetic Blood Penetration Tester Market:
Fully Automatic Medical Masks Synthetic Blood Penetration Testers are advanced devices designed to streamline the testing process, offering high precision and efficiency. These machines are equipped with sophisticated technology that automates the entire testing procedure, from sample preparation to result analysis, minimizing human intervention and error. They are ideal for large-scale mask manufacturers who require consistent and reliable testing to meet high production demands. The fully automatic testers are often integrated with advanced software that allows for real-time data monitoring and analysis, providing users with comprehensive insights into the performance of their masks. This level of automation not only enhances productivity but also ensures compliance with international safety standards, making them a preferred choice for leading manufacturers. On the other hand, Semi-automatic Medical Masks Synthetic Blood Penetration Testers offer a balance between automation and manual operation. These devices require some level of human involvement, particularly in the setup and initiation of tests, but still provide automated data collection and analysis. They are suitable for smaller manufacturers or research facilities that may not have the resources or need for fully automated systems. Despite requiring more manual input, semi-automatic testers still deliver accurate and reliable results, ensuring that masks meet necessary safety standards. Both types of testers play a crucial role in the Global Medical Masks Synthetic Blood Penetration Tester Market, catering to different segments of the industry and contributing to the overall goal of enhancing mask safety and effectiveness. The choice between fully automatic and semi-automatic testers often depends on factors such as budget, production volume, and specific testing requirements. As technology continues to evolve, we can expect further innovations in both categories, offering even more advanced features and capabilities to meet the growing demands of the healthcare industry.
Mask Testing, Medical Testing, Textile Testing, Other in the Global Medical Masks Synthetic Blood Penetration Tester Market:
The Global Medical Masks Synthetic Blood Penetration Tester Market finds its application in various areas, each playing a significant role in ensuring the safety and effectiveness of medical masks. In Mask Testing, these devices are used to evaluate the resistance of masks to synthetic blood penetration, a critical factor in determining their protective capabilities. This testing is essential for manufacturers to ensure that their products meet international safety standards and provide adequate protection against blood-borne pathogens. In Medical Testing, these testers are used to assess the performance of masks in clinical settings, where exposure to blood and other bodily fluids is a common risk. By simulating real-world conditions, these devices help healthcare facilities select the most effective masks for their staff, enhancing overall safety and infection control. In Textile Testing, the focus is on evaluating the materials used in mask production. The synthetic blood penetration testers help determine the suitability of different fabrics and materials, ensuring that they provide the necessary barrier protection without compromising comfort or breathability. This aspect of testing is crucial for manufacturers looking to innovate and improve their products, as it allows them to experiment with new materials and designs while maintaining safety standards. Other applications of these testers include research and development, where they are used to test new mask designs and materials before they are brought to market. This testing is vital for innovation in the industry, as it allows manufacturers to explore new possibilities and improve the performance of their products. Overall, the Global Medical Masks Synthetic Blood Penetration Tester Market plays a crucial role in ensuring the safety and effectiveness of medical masks across various applications, contributing to the overall goal of enhancing healthcare safety and standards.
Global Medical Masks Synthetic Blood Penetration Tester Market Outlook:
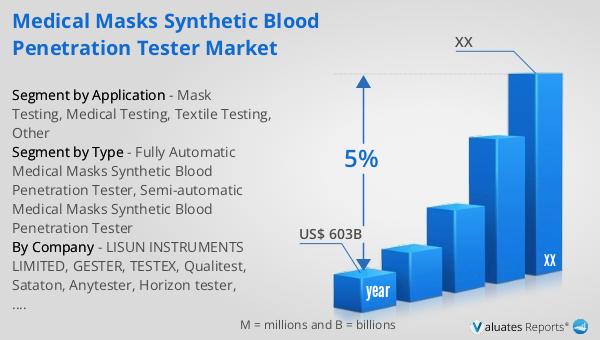
Our research indicates that the global market for medical devices is projected to reach approximately $603 billion in 2023, with an anticipated growth rate of 5% annually over the next six years. This growth is driven by several factors, including technological advancements, increasing healthcare needs, and a growing emphasis on safety and quality standards. The medical device industry encompasses a wide range of products, from diagnostic equipment to surgical instruments, each playing a vital role in modern healthcare. As the demand for these devices continues to rise, driven by an aging population and increasing prevalence of chronic diseases, the market is expected to expand significantly. This growth presents numerous opportunities for manufacturers and investors, as well as challenges in terms of regulatory compliance and competition. The Global Medical Masks Synthetic Blood Penetration Tester Market is a key segment within this broader industry, reflecting the increasing importance of safety and quality assurance in healthcare. As healthcare systems around the world strive to improve patient outcomes and reduce the risk of infection, the demand for reliable and effective testing devices is expected to grow. This market outlook highlights the potential for continued growth and innovation in the medical device industry, driven by a commitment to improving healthcare safety and standards.
| Report Metric | Details |
| Report Name | Medical Masks Synthetic Blood Penetration Tester Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type |
|
| Segment by Application |
|
| Production by Region |
|
| Sales by Region |
|
| By Company | LISUN INSTRUMENTS LIMITED, GESTER, TESTEX, Qualitest, Sataton, Anytester, Horizon tester, Labtech Instrument, HUAZHENG Electric Manufacturing, Qinsun Instruments, Right Instrument, REFOND EQUIPMENT, Bonnin Instrument Technology, UTSTESTER, GBPI tester, TESTRON GROUP, DRICK |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
