What is Global Beta Thalassemia Detection Kits Market?
The Global Beta Thalassemia Detection Kits Market is a specialized segment within the broader medical diagnostics industry. These kits are designed to detect beta thalassemia, a genetic blood disorder that reduces the production of hemoglobin. Hemoglobin is crucial for carrying oxygen in the blood, and its deficiency can lead to severe anemia and other health complications. The detection kits are essential for early diagnosis, which can significantly improve patient outcomes through timely medical intervention. These kits are used in various healthcare settings, including hospitals, specialty clinics, and diagnostic centers. They employ advanced technologies such as PCR (Polymerase Chain Reaction) and other molecular diagnostic techniques to identify mutations in the HBB gene, which is responsible for beta thalassemia. The market for these detection kits is growing due to increasing awareness about genetic disorders, advancements in diagnostic technologies, and the rising prevalence of beta thalassemia, particularly in regions with high carrier rates. The kits come in various formats and sizes to cater to different testing needs and volumes, making them versatile tools in the fight against this debilitating condition.
10 Rxns, 25 Rxns, 50 Rxns, 100 Rxns, Others in the Global Beta Thalassemia Detection Kits Market:
In the Global Beta Thalassemia Detection Kits Market, the kits are available in various reaction sizes, including 10 Rxns, 25 Rxns, 50 Rxns, 100 Rxns, and others. The term "Rxns" stands for reactions, indicating the number of tests that can be performed with a single kit. The 10 Rxns kits are typically used in smaller healthcare settings or for preliminary screenings where the volume of testing is relatively low. These kits are cost-effective and ideal for clinics or laboratories that do not require high-throughput testing. The 25 Rxns kits offer a moderate testing capacity, making them suitable for medium-sized diagnostic centers or specialty clinics that handle a steady flow of patients. These kits strike a balance between cost and efficiency, providing enough tests to meet the needs of a moderately busy facility without the need for frequent reordering. The 50 Rxns kits are designed for larger healthcare institutions or specialized diagnostic centers that conduct a higher volume of tests. These kits are more economical on a per-test basis and reduce the frequency of reordering, making them a practical choice for facilities with a high patient turnover. The 100 Rxns kits are intended for large hospitals, research institutions, or national screening programs where extensive testing is required. These kits offer the highest efficiency and cost savings, allowing for large-scale testing with minimal interruptions. Other formats may include customized kits tailored to specific needs, such as high-throughput automated systems or specialized research applications. These kits may come with additional features or reagents to enhance their functionality and adaptability to various testing environments. The availability of different reaction sizes ensures that healthcare providers can choose the most appropriate kit based on their testing volume, budget, and specific requirements. This flexibility is crucial in addressing the diverse needs of the global healthcare market, where the prevalence of beta thalassemia and the resources available for its detection can vary significantly. By offering a range of options, the Global Beta Thalassemia Detection Kits Market caters to a wide spectrum of healthcare providers, from small clinics to large hospitals and research institutions, ensuring that accurate and timely diagnosis is accessible to all.
Hospitals & Specialty Clinics, Diagnostics Centers, Others in the Global Beta Thalassemia Detection Kits Market:
The usage of Global Beta Thalassemia Detection Kits Market spans across various healthcare settings, including hospitals and specialty clinics, diagnostic centers, and other facilities. In hospitals and specialty clinics, these detection kits play a crucial role in the early diagnosis and management of beta thalassemia. Hospitals often have the infrastructure and resources to conduct comprehensive genetic testing, and the availability of these kits allows for timely identification of affected individuals. Early diagnosis is essential for initiating appropriate treatment plans, which may include regular blood transfusions, iron chelation therapy, and other supportive measures. Specialty clinics, particularly those focused on hematology and genetic disorders, rely on these kits to provide specialized care to patients with beta thalassemia. These clinics often serve as referral centers for complex cases, and the use of advanced detection kits ensures accurate diagnosis and effective management of the condition. Diagnostic centers, on the other hand, are dedicated facilities that focus on conducting various medical tests, including genetic screenings. These centers often handle a high volume of samples and require reliable and efficient detection kits to meet the demand. The use of beta thalassemia detection kits in diagnostic centers ensures that patients receive accurate and timely results, which are critical for making informed medical decisions. Additionally, these centers may collaborate with hospitals and clinics to provide comprehensive diagnostic services, further enhancing the overall quality of care. Other facilities that utilize beta thalassemia detection kits include research institutions, public health programs, and community health centers. Research institutions use these kits to study the genetic basis of beta thalassemia, develop new diagnostic methods, and explore potential treatments. Public health programs, particularly in regions with high carrier rates, use these kits for population screening and genetic counseling to prevent the spread of the disorder. Community health centers, which often serve underserved populations, rely on these kits to provide essential diagnostic services to individuals who may not have access to specialized healthcare facilities. The widespread use of beta thalassemia detection kits across various healthcare settings underscores their importance in the early diagnosis and management of this genetic disorder. By providing accurate and timely results, these kits enable healthcare providers to deliver effective care and improve patient outcomes.
Global Beta Thalassemia Detection Kits Market Outlook:
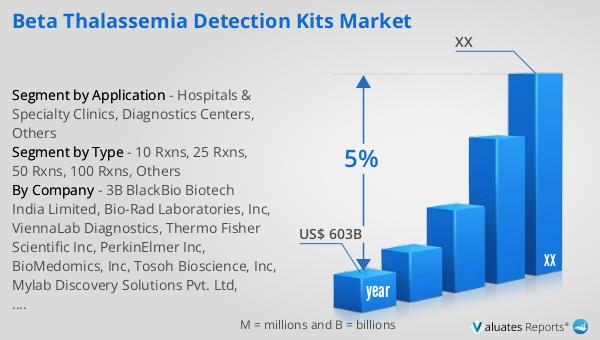
According to our research, the global market for medical devices is projected to reach approximately US$ 603 billion by the year 2023, with an anticipated growth rate of 5% CAGR over the next six years. This growth is driven by several factors, including advancements in medical technology, increasing prevalence of chronic diseases, and rising demand for early and accurate diagnostic tools. The medical device industry encompasses a wide range of products, from simple instruments to complex machinery, all designed to improve patient care and outcomes. The continuous innovation in this field has led to the development of more efficient, reliable, and user-friendly devices, which are essential for modern healthcare. The growing awareness about the importance of early diagnosis and preventive care has also contributed to the expansion of the medical device market. As healthcare systems worldwide strive to improve their services, the demand for advanced diagnostic and therapeutic devices continues to rise. This trend is particularly evident in emerging markets, where improving healthcare infrastructure and increasing healthcare expenditure are driving the adoption of new technologies. The projected growth of the medical device market reflects the ongoing efforts to enhance healthcare delivery and address the evolving needs of patients and healthcare providers.
| Report Metric | Details |
| Report Name | Beta Thalassemia Detection Kits Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | 3B BlackBio Biotech India Limited, Bio-Rad Laboratories, Inc, ViennaLab Diagnostics, Thermo Fisher Scientific Inc, PerkinElmer Inc, BioMedomics, Inc, Tosoh Bioscience, Inc, Mylab Discovery Solutions Pvt. Ltd, HiMedia, Devyse, GENETEK Biopharma GmbH, RIDACOM, BGI Genomics Co. LTD, Guangdong Hybribio Biotech CO.,Ltd |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
