What is Global Rivaroxaban API Market?
The Global Rivaroxaban API Market refers to the worldwide market for the active pharmaceutical ingredient (API) used in the production of Rivaroxaban, a medication primarily used to prevent and treat blood clots. Rivaroxaban is an anticoagulant that works by inhibiting Factor Xa, an essential component in the blood clotting process. This market encompasses the production, distribution, and sale of Rivaroxaban API to pharmaceutical companies that manufacture the final drug product. The demand for Rivaroxaban API is driven by the increasing prevalence of conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), and atrial fibrillation, which require anticoagulation therapy. The market is also influenced by factors such as regulatory approvals, patent expirations, and the introduction of generic versions of the drug. As the global population ages and the incidence of cardiovascular diseases rises, the need for effective anticoagulant therapies like Rivaroxaban is expected to grow, thereby driving the demand for its API.
Purity≥98%, Purity<98% in the Global Rivaroxaban API Market:
In the Global Rivaroxaban API Market, the purity of the API is a critical factor that significantly impacts its efficacy and safety. Purity levels are generally categorized into two main groups: Purity≥98% and Purity<98%. APIs with a purity of 98% or higher are considered to be of high quality and are preferred for pharmaceutical manufacturing due to their superior performance and lower risk of impurities that could cause adverse effects. High-purity APIs ensure that the final drug product meets stringent regulatory standards and provides consistent therapeutic outcomes. On the other hand, APIs with a purity of less than 98% may contain higher levels of impurities, which can affect the drug's safety and effectiveness. These impurities can arise from various sources, including the raw materials used, the manufacturing process, and storage conditions. While lower-purity APIs may be less expensive, they pose a higher risk of causing side effects or therapeutic failures, making them less desirable for use in high-stakes medical treatments. The choice between high-purity and lower-purity APIs often depends on the specific requirements of the pharmaceutical company and the intended use of the final drug product. Regulatory agencies around the world, such as the FDA in the United States and the EMA in Europe, have stringent guidelines for the acceptable purity levels of APIs used in drug manufacturing. These guidelines are designed to ensure that the final drug products are safe, effective, and of high quality. In the context of the Global Rivaroxaban API Market, maintaining high purity levels is particularly important given the drug's role in preventing life-threatening conditions like blood clots. Pharmaceutical companies invest heavily in quality control measures to ensure that their APIs meet or exceed the required purity standards. This includes rigorous testing and validation processes to detect and eliminate impurities. Advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and mass spectrometry, are commonly used to assess the purity of APIs. These techniques provide precise and accurate measurements, enabling manufacturers to maintain high standards of quality. In addition to regulatory compliance, high-purity APIs also offer competitive advantages in the market. Pharmaceutical companies that can consistently produce high-purity APIs are more likely to gain the trust of healthcare providers and patients, leading to increased market share and profitability. Furthermore, high-purity APIs can enhance the overall performance of the final drug product, resulting in better patient outcomes and reduced healthcare costs. In summary, the purity of Rivaroxaban API is a crucial factor that influences its market dynamics. High-purity APIs are preferred for their superior quality and safety, while lower-purity APIs may pose risks due to higher levels of impurities. Regulatory guidelines and advanced analytical techniques play a vital role in ensuring that APIs meet the required purity standards. Pharmaceutical companies that prioritize high-purity APIs are better positioned to succeed in the competitive Global Rivaroxaban API Market.
Rivaroxaban Tablets, Others in the Global Rivaroxaban API Market:
The Global Rivaroxaban API Market finds its primary application in the production of Rivaroxaban tablets, which are widely used for the prevention and treatment of various thromboembolic disorders. Rivaroxaban tablets are prescribed to patients with conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), and atrial fibrillation, where the risk of blood clots is significantly elevated. These tablets offer a convenient oral administration route, making them a preferred choice for both patients and healthcare providers. The efficacy of Rivaroxaban tablets in preventing blood clots has been well-documented in numerous clinical trials, leading to their widespread adoption in clinical practice. In addition to Rivaroxaban tablets, the Global Rivaroxaban API Market also caters to other formulations and applications. For instance, Rivaroxaban API is used in the development of extended-release formulations, which provide a sustained release of the drug over an extended period. This can be particularly beneficial for patients who require long-term anticoagulation therapy, as it reduces the frequency of dosing and improves patient compliance. Moreover, Rivaroxaban API is also utilized in combination therapies, where it is combined with other anticoagulants or antiplatelet agents to enhance therapeutic outcomes. These combination therapies are often used in complex clinical scenarios where a single anticoagulant may not be sufficient to manage the patient's condition effectively. The versatility of Rivaroxaban API in various formulations and therapeutic regimens underscores its importance in the global pharmaceutical market. Another significant application of Rivaroxaban API is in the development of generic versions of the drug. As the patents for Rivaroxaban expire, generic pharmaceutical companies are entering the market with their versions of the drug, which are typically more affordable than the branded product. The availability of generic Rivaroxaban is expected to increase access to this essential medication, particularly in low- and middle-income countries where the cost of branded drugs can be prohibitive. The production of generic Rivaroxaban requires high-quality API to ensure that the final product meets the same standards of safety and efficacy as the original branded drug. This has led to increased demand for Rivaroxaban API from generic manufacturers, further driving the growth of the Global Rivaroxaban API Market. In summary, the Global Rivaroxaban API Market plays a crucial role in the production of Rivaroxaban tablets and other formulations used in the prevention and treatment of thromboembolic disorders. The versatility of Rivaroxaban API in various therapeutic regimens and its importance in the development of generic versions of the drug highlight its significance in the global pharmaceutical landscape. As the demand for effective anticoagulant therapies continues to rise, the Global Rivaroxaban API Market is expected to grow, driven by the need for high-quality API to support the production of safe and effective medications.
Global Rivaroxaban API Market Outlook:
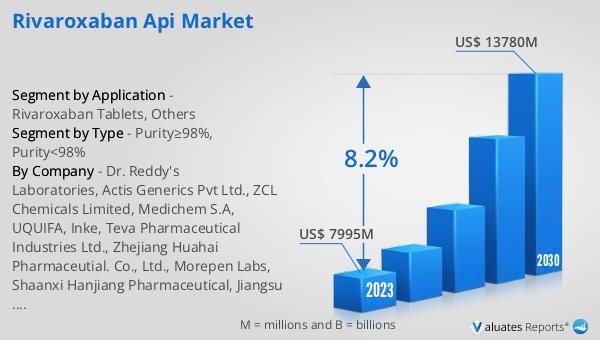
The global Rivaroxaban API market was valued at US$ 7995 million in 2023 and is anticipated to reach US$ 13780 million by 2030, witnessing a CAGR of 8.2% during the forecast period 2024-2030. The global pharmaceutical market was valued at 1475 billion USD in 2022 and is expected to grow at a CAGR of 5% over the next six years. In comparison, the chemical drug market was estimated to increase from 1005 billion USD in 2018 to 1094 billion USD in 2022. This data highlights the significant growth potential of the Rivaroxaban API market within the broader pharmaceutical and chemical drug markets. The increasing prevalence of cardiovascular diseases and the growing demand for effective anticoagulant therapies are key factors driving the growth of the Rivaroxaban API market. As the market continues to expand, pharmaceutical companies are likely to invest in the development and production of high-quality Rivaroxaban API to meet the rising demand for this essential medication.
| Report Metric | Details |
| Report Name | Rivaroxaban API Market |
| Accounted market size in 2023 | US$ 7995 million |
| Forecasted market size in 2030 | US$ 13780 million |
| CAGR | 8.2% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Production by Region |
|
| Consumption by Region |
|
| By Company | Dr. Reddy's Laboratories, Actis Generics Pvt Ltd., ZCL Chemicals Limited, Medichem S.A, UQUIFA, Inke, Teva Pharmaceutical Industries Ltd., Zhejiang Huahai Pharmaceutial. Co., Ltd., Morepen Labs, Shaanxi Hanjiang Pharmaceutical, Jiangsu Jiayi, Shanghai Haoyuan, Nanjing Hicin |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
