What is Global Mycoplasma Detection Kit Market?
The Global Mycoplasma Detection Kit Market refers to the worldwide industry focused on the production and distribution of kits designed to detect mycoplasma contamination. Mycoplasmas are a type of bacteria that lack a cell wall, making them resistant to many common antibiotics. They can contaminate cell cultures, leading to inaccurate experimental results and potentially harmful effects in clinical settings. These detection kits are essential tools in various fields, including pharmaceuticals, biotechnology, and academic research, as they help ensure the purity and reliability of cell cultures. The market for these kits is driven by the increasing demand for biopharmaceutical products, the growing emphasis on quality control in research and manufacturing, and the rising awareness about the importance of detecting mycoplasma contamination. The kits typically use methods such as PCR (Polymerase Chain Reaction), ELISA (Enzyme-Linked Immunosorbent Assay), and direct culture techniques to identify the presence of mycoplasma. As the biotechnology and pharmaceutical industries continue to expand, the need for reliable mycoplasma detection solutions is expected to grow, making this market a critical component of the global healthcare and research infrastructure.
Colloidal Gold Type, Enzyme Linked Immunosorbent in the Global Mycoplasma Detection Kit Market:
Colloidal Gold Type and Enzyme-Linked Immunosorbent Assay (ELISA) are two prominent methods used in the Global Mycoplasma Detection Kit Market. The Colloidal Gold Type method involves the use of gold nanoparticles conjugated with antibodies specific to mycoplasma antigens. When a sample containing mycoplasma is introduced, the antigens bind to the antibodies on the gold nanoparticles, forming a visible line on a test strip. This method is known for its simplicity, rapid results, and ease of use, making it suitable for point-of-care testing and field applications. It does not require sophisticated equipment or extensive training, which makes it accessible to a wide range of users. On the other hand, the Enzyme-Linked Immunosorbent Assay (ELISA) is a more complex and sensitive method that involves the use of enzymes and antibodies to detect mycoplasma antigens. In an ELISA test, a sample is added to a plate coated with antibodies specific to mycoplasma antigens. If mycoplasma is present, the antigens will bind to the antibodies on the plate. A secondary antibody conjugated with an enzyme is then added, which binds to the antigen-antibody complex. When a substrate is introduced, the enzyme catalyzes a reaction that produces a detectable signal, usually a color change. ELISA is highly sensitive and can detect low levels of mycoplasma contamination, making it ideal for applications where accuracy is critical. However, it requires specialized equipment and trained personnel, which can limit its use in some settings. Both methods have their advantages and are chosen based on the specific needs of the user. The Colloidal Gold Type is favored for its speed and simplicity, while ELISA is preferred for its sensitivity and accuracy. The choice between these methods depends on factors such as the required sensitivity, available resources, and the specific application. In the context of the Global Mycoplasma Detection Kit Market, both methods play a crucial role in ensuring the reliability and safety of cell cultures used in research, manufacturing, and clinical settings. As the demand for biopharmaceutical products and quality control continues to rise, the importance of these detection methods is expected to grow, driving innovation and development in the market.
Hospital, Clinics, Testing Institutions in the Global Mycoplasma Detection Kit Market:
The Global Mycoplasma Detection Kit Market finds significant usage in hospitals, clinics, and testing institutions, each with its unique requirements and applications. In hospitals, mycoplasma detection kits are crucial for ensuring the safety and efficacy of cell-based therapies and other biopharmaceutical products. Hospitals often engage in clinical trials and research activities that involve the use of cell cultures. Mycoplasma contamination can compromise the integrity of these cultures, leading to inaccurate results and potentially harmful outcomes for patients. By using mycoplasma detection kits, hospitals can maintain the purity of their cell cultures, ensuring that their research and treatments are based on reliable data. Clinics, on the other hand, may use mycoplasma detection kits for diagnostic purposes. Mycoplasma infections can cause a range of health issues, including respiratory and urogenital infections. Rapid and accurate detection of mycoplasma is essential for effective treatment and management of these infections. Colloidal Gold Type kits, with their simplicity and quick results, are particularly useful in clinical settings where timely diagnosis is critical. Testing institutions, such as research laboratories and quality control facilities, rely heavily on mycoplasma detection kits to ensure the accuracy and reliability of their experiments and products. These institutions often work with a wide variety of cell cultures, and mycoplasma contamination can lead to significant setbacks in their research and development efforts. ELISA kits, with their high sensitivity and accuracy, are commonly used in these settings to detect even low levels of mycoplasma contamination. By regularly testing their cell cultures, testing institutions can prevent contamination-related issues and maintain the integrity of their work. Overall, the usage of mycoplasma detection kits in hospitals, clinics, and testing institutions highlights the critical role these tools play in ensuring the safety, efficacy, and reliability of cell-based research and treatments. As the demand for biopharmaceutical products and advanced medical treatments continues to grow, the importance of mycoplasma detection in these settings is expected to increase, driving further innovation and development in the Global Mycoplasma Detection Kit Market.
Global Mycoplasma Detection Kit Market Outlook:
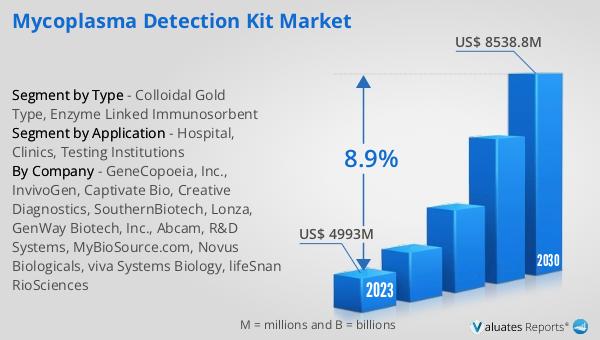
The global Mycoplasma Detection Kit market was valued at US$ 4993 million in 2023 and is anticipated to reach US$ 8538.8 million by 2030, witnessing a CAGR of 8.9% during the forecast period 2024-2030. This significant growth reflects the increasing demand for reliable and efficient mycoplasma detection solutions across various sectors, including pharmaceuticals, biotechnology, and clinical research. The rising awareness about the detrimental effects of mycoplasma contamination on cell cultures and the subsequent impact on research and product development is driving the adoption of these kits. Additionally, the growing emphasis on quality control and regulatory compliance in the biopharmaceutical industry is further propelling the market. As more institutions recognize the importance of maintaining contamination-free cell cultures, the demand for advanced mycoplasma detection methods is expected to rise. This market outlook underscores the critical role that mycoplasma detection kits play in ensuring the integrity and reliability of cell-based research and products, highlighting the ongoing need for innovation and development in this field.
| Report Metric | Details |
| Report Name | Mycoplasma Detection Kit Market |
| Accounted market size in 2023 | US$ 4993 million |
| Forecasted market size in 2030 | US$ 8538.8 million |
| CAGR | 8.9% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | GeneCopoeia, Inc., InvivoGen, Captivate Bio, Creative Diagnostics, SouthernBiotech, Lonza, GenWay Biotech, Inc., Abcam, R&D Systems, MyBioSource.com, Novus Biologicals, viva Systems Biology, lifeSnan RioSciences |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
