What is Global Medical Device Process Validation Services Market?
The Global Medical Device Process Validation Services Market is a crucial segment within the healthcare industry, focusing on ensuring that medical devices are manufactured, packaged, cleaned, and sterilized according to stringent regulatory standards. This market encompasses a range of services designed to validate the processes used in the production of medical devices, ensuring they meet the required safety and efficacy standards before reaching the end user. With the increasing demand for medical devices worldwide, driven by an aging population, technological advancements, and a growing focus on healthcare quality, the need for comprehensive process validation services is more critical than ever. These services not only help manufacturers comply with regulatory requirements but also ensure the reliability and safety of medical devices, ultimately contributing to better patient outcomes. As the healthcare industry continues to evolve, the Global Medical Device Process Validation Services Market plays a pivotal role in supporting the development and distribution of safe, effective medical devices.

Medical Device Manufacturing And Packaging Validation, Cleaning And Sterilization Validation, Automation / Control System Validation, Others in the Global Medical Device Process Validation Services Market:
The Global Medical Device Process Validation Services Market encompasses several key areas, including Medical Device Manufacturing and Packaging Validation, Cleaning and Sterilization Validation, Automation/Control System Validation, among others. Each of these areas plays a vital role in ensuring that medical devices are produced and maintained according to the highest standards. Manufacturing and Packaging Validation services ensure that the design and production processes of medical devices, as well as their packaging, meet specific regulatory and quality standards, minimizing risks associated with device failures or contamination. Cleaning and Sterilization Validation is equally critical, as it verifies the effectiveness of cleaning and sterilization processes to prevent infections and ensure device safety. Automation/Control System Validation focuses on ensuring that automated systems used in the manufacturing process operate reliably and consistently, further ensuring product quality. Other services within this market may include software validation, equipment validation, and more, each addressing different aspects of the medical device production process. Together, these services form a comprehensive ecosystem aimed at ensuring medical devices are safe, effective, and compliant with global regulatory standards, ultimately supporting the delivery of high-quality healthcare.
Pharmaceutical Industry, Chemical Industry, Medical Industry, Others in the Global Medical Device Process Validation Services Market:
The Global Medical Device Process Validation Services Market finds its application across various industries, notably in the Pharmaceutical, Chemical, Medical, and other sectors. In the Pharmaceutical Industry, these services are indispensable for validating processes involved in the production of drug delivery devices, ensuring they meet stringent regulatory standards for safety and efficacy. The Chemical Industry also benefits from these services, particularly in the validation of devices used in the production and handling of chemicals, where safety and contamination prevention are paramount. The Medical Industry, encompassing hospitals, clinics, and other healthcare facilities, relies on validated medical devices for diagnostics, treatment, and patient care, making these services critical for patient safety and care quality. Other sectors, including biotechnology, food and beverage, and cosmetics, also utilize these services to ensure their products meet regulatory requirements and safety standards. The widespread application of the Global Medical Device Process Validation Services Market underscores its importance in maintaining high standards of safety and quality across industries reliant on medical devices and related technologies.
Global Medical Device Process Validation Services Market Outlook:
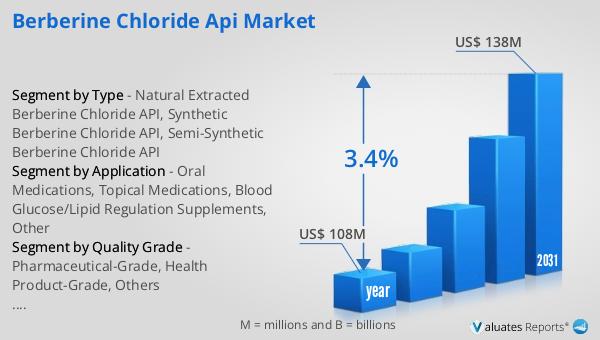
Our research indicates that the global market for medical devices is currently valued at approximately US$ 603 billion as of the year 2023. Over the next six years, this market is expected to grow at a compound annual growth rate (CAGR) of 5%. This growth trajectory underscores the increasing demand for medical devices across various healthcare sectors, driven by technological advancements, an aging global population, and a heightened focus on healthcare quality and outcomes. The expansion of the medical device market reflects the critical role these devices play in diagnosing, treating, and managing a wide range of health conditions, from chronic diseases to acute medical emergencies. As the market continues to grow, the need for comprehensive process validation services to ensure the safety, efficacy, and quality of these devices becomes increasingly important, highlighting the vital role of the Global Medical Device Process Validation Services Market in supporting the broader healthcare industry's objectives.
| Report Metric | Details |
| Report Name | Medical Device Process Validation Services Market |
| Accounted market size in year | US$ 603 billion |
| CAGR | 5% |
| Base Year | year |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | EMERGO, Master Control, Operon Strategist, BioTeknica, BMP Medical, RS NESS, VALGENESIS, Integrated Commissioning & Qualification Consultants, Corp., Riverside Medical Packaging, Sterling Medical Devices, LLS Health, Elos Medtech, Shimadzu |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |