What is Global Clinical Trial Supply and Logistic for Pharmaceutical Market?
The Global Clinical Trial Supply and Logistic for Pharmaceutical Market is a crucial aspect of the healthcare industry. It involves the management and coordination of various resources, including drugs, medical devices, and other supplies, required for conducting clinical trials. These trials are essential for testing the safety and efficacy of new drugs and treatments before they are approved for public use. The market encompasses various stages, from the initial planning and design of the trial, to the procurement and distribution of supplies, and finally, the collection and analysis of data. The process is complex and requires meticulous planning and execution to ensure the integrity of the trial and the safety of the participants. The market is driven by the increasing demand for new drugs and treatments, the growing prevalence of chronic diseases, and the rising investment in research and development activities by pharmaceutical companies.
Logistics & Distribution, Manufacturing and Packaging, Supply Chain Management, Other in the Global Clinical Trial Supply and Logistic for Pharmaceutical Market:
The Global Clinical Trial Supply and Logistic for Pharmaceutical Market is segmented into various components, including Logistics & Distribution, Manufacturing and Packaging, Supply Chain Management, and others. Logistics & Distribution involves the transportation of supplies from the manufacturers to the trial sites. This requires careful planning and coordination to ensure timely delivery and prevent any disruptions in the trial. Manufacturing and Packaging involve the production and packaging of the trial supplies, ensuring they meet the required standards and regulations. Supply Chain Management is crucial for managing the flow of supplies and information throughout the trial. It involves coordinating with various stakeholders, including manufacturers, distributors, trial sites, and regulatory authorities. Other components include data management, quality control, and regulatory compliance, among others. Each component plays a vital role in the successful execution of a clinical trial.
Phase I, Phase II, Phase III in the Global Clinical Trial Supply and Logistic for Pharmaceutical Market:
The Global Clinical Trial Supply and Logistic for Pharmaceutical Market is used in various phases of clinical trials, including Phase I, Phase II, and Phase III. Phase I trials are the first stage of testing in humans and involve a small number of healthy volunteers. The primary aim is to assess the safety and dosage of the new drug. The supply and logistic requirements for this phase are relatively small but crucial for the success of the trial. Phase II trials involve a larger group of patients and aim to assess the efficacy and side effects of the drug. The supply and logistic requirements for this phase are more extensive and complex, requiring careful planning and management. Phase III trials are the final stage before the drug is approved for public use. They involve a large number of patients and aim to confirm the efficacy of the drug and monitor its side effects in a larger population. The supply and logistic requirements for this phase are the most complex and extensive, requiring meticulous planning and execution.
Global Clinical Trial Supply and Logistic for Pharmaceutical Market Outlook:
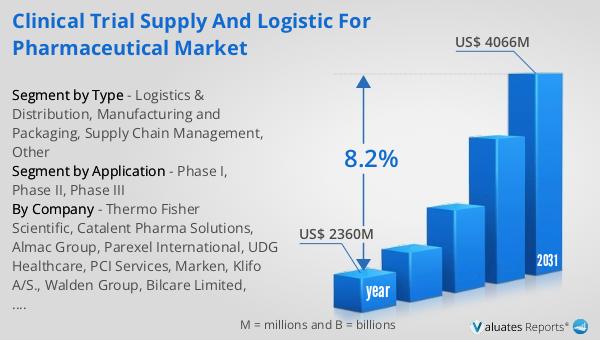
The Global Clinical Trial Supply and Logistic for Pharmaceutical Market has shown significant growth in recent years. In 2023, the market was valued at US$ 2018 million and is expected to reach US$ 3526.1 million by 2030, growing at a CAGR of 8.2% during the forecast period 2024-2030. The market is dominated by key players such as Thermo Fisher Scientific, Catalent Pharma Solutions, Almac Group, and Parexel International, who collectively hold about 70% of the market share. The Americas region is the largest market, accounting for about 60% of the global market share. Phase III trials are the main application of the market, accounting for over 50% of the market share. The growth of the market is driven by the increasing demand for new drugs and treatments, the growing prevalence of chronic diseases, and the rising investment in research and development activities by pharmaceutical companies.
| Report Metric | Details |
| Report Name | Clinical Trial Supply and Logistic for Pharmaceutical Market |
| Accounted market size in 2023 | US$ 2018 million |
| Forecasted market size in 2030 | US$ 3526.1 million |
| CAGR | 8.2% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Thermo Fisher Scientific, Catalent Pharma Solutions, Almac Group, Parexel International, UDG Healthcare, PCI Services, Marken, Klifo A/S., Walden Group, Bilcare Limited, Biocair |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |