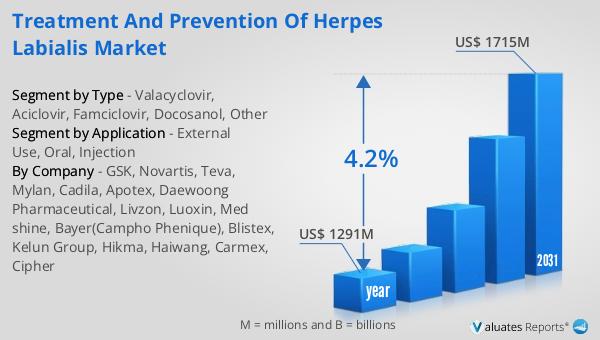
What is Global Diagnostic Biomarkers Market?
The Global Diagnostic Biomarkers Market is a rapidly evolving sector within the healthcare industry, focusing on the identification and utilization of biomarkers for diagnostic purposes. Biomarkers are biological molecules found in blood, other body fluids, or tissues that signal a normal or abnormal process, or a condition or disease. They are crucial in the early detection, diagnosis, and monitoring of diseases, offering a more personalized approach to medicine. This market is driven by the increasing prevalence of chronic diseases, advancements in genomics and proteomics, and the growing demand for personalized medicine. The integration of biomarkers into diagnostic procedures enhances the accuracy and efficiency of disease detection, leading to better patient outcomes. Moreover, technological advancements and increased funding for research and development are propelling the growth of this market. As healthcare systems worldwide strive for more precise and effective diagnostic tools, the Global Diagnostic Biomarkers Market is poised to play a pivotal role in transforming patient care and treatment strategies.
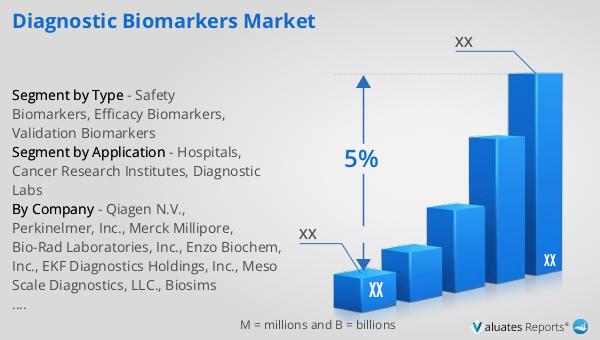
Safety Biomarkers, Efficacy Biomarkers, Validation Biomarkers in the Global Diagnostic Biomarkers Market:
Safety biomarkers, efficacy biomarkers, and validation biomarkers are integral components of the Global Diagnostic Biomarkers Market, each serving distinct yet interconnected roles. Safety biomarkers are primarily used to assess the safety profile of a drug or treatment. They help in identifying any adverse effects or toxicities that may arise during clinical trials or treatment regimens. By providing early warnings about potential side effects, safety biomarkers play a crucial role in ensuring patient safety and guiding the development of safer therapeutic interventions. Efficacy biomarkers, on the other hand, are used to evaluate the effectiveness of a treatment. They help in determining whether a drug is producing the desired therapeutic effect in patients. By measuring the biological response to a treatment, efficacy biomarkers enable researchers and clinicians to make informed decisions about the continuation, modification, or cessation of a treatment regimen. This not only enhances the therapeutic outcomes but also optimizes the use of healthcare resources. Validation biomarkers are essential for confirming the reliability and accuracy of other biomarkers. They are used to validate the findings obtained from safety and efficacy biomarkers, ensuring that the results are consistent and reproducible. Validation biomarkers help in establishing the credibility of biomarker-based diagnostic tests, thereby facilitating their integration into clinical practice. In the context of the Global Diagnostic Biomarkers Market, these biomarkers collectively contribute to the development of more precise and personalized diagnostic tools. They enable healthcare providers to tailor treatments to individual patients, improving the overall quality of care. Furthermore, the use of these biomarkers in clinical trials accelerates the drug development process, reducing the time and cost associated with bringing new therapies to market. As the demand for personalized medicine continues to grow, the role of safety, efficacy, and validation biomarkers in the Global Diagnostic Biomarkers Market is expected to become increasingly significant.
Hospitals, Cancer Research Institutes, Diagnostic Labs in the Global Diagnostic Biomarkers Market:
The Global Diagnostic Biomarkers Market finds extensive application in various healthcare settings, including hospitals, cancer research institutes, and diagnostic labs. In hospitals, diagnostic biomarkers are used to enhance the accuracy and speed of disease diagnosis. They enable healthcare professionals to identify diseases at an early stage, allowing for timely intervention and improved patient outcomes. For instance, biomarkers can be used to detect cardiac events, infections, or metabolic disorders, providing critical information that guides treatment decisions. In cancer research institutes, diagnostic biomarkers are pivotal in advancing cancer research and treatment. They help in identifying specific genetic mutations or protein expressions associated with different types of cancer, facilitating the development of targeted therapies. By understanding the molecular basis of cancer, researchers can design more effective treatment strategies that minimize side effects and improve survival rates. Diagnostic biomarkers also play a crucial role in monitoring disease progression and response to treatment, enabling researchers to refine therapeutic approaches and improve patient care. In diagnostic labs, biomarkers are used to develop and validate diagnostic tests that are essential for disease screening and monitoring. These tests provide valuable insights into a patient's health status, helping clinicians to make informed decisions about treatment and management. The use of biomarkers in diagnostic labs enhances the precision and reliability of diagnostic tests, leading to more accurate and timely diagnoses. As the demand for personalized medicine continues to rise, the application of diagnostic biomarkers in hospitals, cancer research institutes, and diagnostic labs is expected to expand, driving the growth of the Global Diagnostic Biomarkers Market.
Global Diagnostic Biomarkers Market Outlook:
The outlook for the Global Diagnostic Biomarkers Market can be contextualized by examining the broader pharmaceutical and chemical drug markets. In 2022, the global pharmaceutical market was valued at approximately 1,475 billion USD, with an anticipated compound annual growth rate (CAGR) of 5% over the next six years. This growth is indicative of the increasing demand for innovative healthcare solutions and the rising prevalence of chronic diseases worldwide. In comparison, the chemical drug market experienced a growth trajectory from 1,005 billion USD in 2018 to an estimated 1,094 billion USD in 2022. This growth reflects the ongoing advancements in drug development and the increasing adoption of chemical drugs in various therapeutic areas. The expansion of these markets underscores the importance of diagnostic biomarkers in enhancing the precision and effectiveness of medical treatments. As the pharmaceutical and chemical drug markets continue to grow, the demand for diagnostic biomarkers is expected to rise, driven by the need for more accurate and personalized diagnostic tools. This trend highlights the critical role of the Global Diagnostic Biomarkers Market in shaping the future of healthcare and improving patient outcomes.
| Report Metric | Details |
| Report Name | Diagnostic Biomarkers Market |
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Qiagen N.V., Perkinelmer, Inc., Merck Millipore, Bio-Rad Laboratories, Inc., Enzo Biochem, Inc., EKF Diagnostics Holdings, Inc., Meso Scale Diagnostics, LLC., Biosims Technologies Sas, Cisbio Bioassays, Signosis, Inc, Banyan Biomarkers, Inc, Biomedical Corp |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |