What is Global e-clinical Trials Market?
The Global e-clinical Trials Market represents a transformative shift in how clinical trials are conducted worldwide. This market encompasses a range of digital solutions designed to streamline and enhance the efficiency of clinical trials, which are essential for developing new medical treatments and drugs. By leveraging advanced technologies, e-clinical trials aim to reduce the time and cost associated with traditional clinical trials while improving data accuracy and regulatory compliance. These digital solutions include electronic data capture, remote monitoring, and real-time data analysis, which collectively facilitate more agile and adaptive trial designs. The global e-clinical trials market is driven by the increasing complexity of clinical trials, the need for more efficient data management, and the growing demand for personalized medicine. As the healthcare industry continues to embrace digital transformation, the adoption of e-clinical trial solutions is expected to rise, offering significant benefits to pharmaceutical companies, research organizations, and ultimately, patients. By enabling faster and more reliable clinical trials, the global e-clinical trials market plays a crucial role in accelerating the development of new therapies and improving patient outcomes worldwide.
Clinical Data Management System(CDMS) Solutions, Clinical Trial Management System(CTMS) Solutions, Electronic Clinical Outcomes Assessment(eCOA) Solutions, Randomization and Trial Supply Management(RTSM) Solutions, Other in the Global e-clinical Trials Market:
Clinical Data Management System (CDMS) Solutions are pivotal in the Global e-clinical Trials Market, serving as the backbone for collecting, cleaning, and managing clinical trial data. These systems ensure that data is accurate, consistent, and compliant with regulatory standards, which is crucial for the integrity of clinical trials. CDMS solutions automate many of the manual processes involved in data management, reducing the risk of errors and enabling faster data analysis. This efficiency is particularly important in large-scale trials where vast amounts of data are generated. Clinical Trial Management System (CTMS) Solutions, on the other hand, focus on the operational aspects of clinical trials. They provide tools for planning, tracking, and managing the various components of a trial, such as site selection, patient recruitment, and resource allocation. CTMS solutions help streamline trial management, improve communication among stakeholders, and ensure that trials are conducted on time and within budget. Electronic Clinical Outcomes Assessment (eCOA) Solutions are designed to capture patient-reported outcomes and other clinical data directly from patients. By using electronic devices such as tablets and smartphones, eCOA solutions enhance data accuracy and patient engagement, providing real-time insights into patient experiences and treatment efficacy. Randomization and Trial Supply Management (RTSM) Solutions are critical for ensuring that clinical trials are conducted in a scientifically rigorous manner. These solutions manage the random assignment of treatments to participants and oversee the supply chain for trial materials, ensuring that the right products are delivered to the right locations at the right times. This is essential for maintaining the integrity of the trial and ensuring that results are reliable. Other solutions in the e-clinical trials market include electronic data capture (EDC) systems, which facilitate the collection and storage of trial data in a digital format, and remote monitoring tools, which allow for the oversight of trial sites without the need for physical visits. Together, these solutions form a comprehensive ecosystem that supports the efficient and effective conduct of clinical trials, ultimately accelerating the development of new medical treatments.
Medical Laboratory, Hospital, Pharmaceutical Company, Other in the Global e-clinical Trials Market:
The Global e-clinical Trials Market finds extensive application across various sectors, including medical laboratories, hospitals, pharmaceutical companies, and other healthcare-related entities. In medical laboratories, e-clinical trial solutions streamline the process of data collection and analysis, enabling researchers to efficiently manage large volumes of data generated during trials. This is particularly beneficial in complex studies that require precise data handling and analysis. By automating data management tasks, e-clinical solutions reduce the likelihood of errors and enhance the reliability of trial results. Hospitals also benefit significantly from e-clinical trial solutions, as they facilitate better coordination and communication among different departments involved in clinical research. These solutions enable hospitals to efficiently manage patient recruitment, monitor patient progress, and ensure compliance with regulatory requirements. By providing real-time access to trial data, e-clinical solutions help healthcare professionals make informed decisions and improve patient care. Pharmaceutical companies are among the primary users of e-clinical trial solutions, as these tools are essential for the development and testing of new drugs. E-clinical solutions enable pharmaceutical companies to conduct trials more efficiently, reducing the time and cost associated with bringing new drugs to market. By improving data accuracy and trial management, these solutions help pharmaceutical companies meet regulatory standards and accelerate the approval process for new treatments. Other entities, such as contract research organizations (CROs) and academic institutions, also utilize e-clinical trial solutions to enhance their research capabilities. CROs, in particular, rely on these solutions to manage multiple trials simultaneously, ensuring that each study is conducted according to the highest standards of quality and compliance. Academic institutions benefit from e-clinical solutions by gaining access to advanced tools for data analysis and trial management, enabling them to conduct cutting-edge research and contribute to the advancement of medical science. Overall, the Global e-clinical Trials Market plays a vital role in supporting the diverse needs of the healthcare industry, driving innovation, and improving patient outcomes.
Global e-clinical Trials Market Outlook:
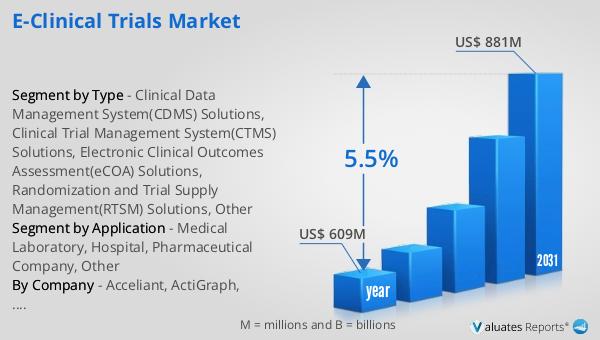
The global market for e-clinical trials was valued at approximately $609 million in 2024, and it is anticipated to grow significantly, reaching an estimated size of $881 million by 2031. This growth trajectory reflects a compound annual growth rate (CAGR) of 5.5% over the forecast period. The increasing adoption of digital technologies in clinical trials is a key driver of this market expansion. As pharmaceutical companies and research organizations seek more efficient and cost-effective ways to conduct clinical trials, the demand for e-clinical solutions continues to rise. These solutions offer numerous advantages, including improved data accuracy, enhanced trial management, and faster regulatory compliance. By streamlining the clinical trial process, e-clinical solutions enable organizations to bring new treatments to market more quickly, ultimately benefiting patients worldwide. The projected growth of the e-clinical trials market underscores the importance of digital transformation in the healthcare industry and highlights the potential for continued innovation in clinical research. As the market evolves, stakeholders across the healthcare ecosystem are likely to increasingly rely on e-clinical solutions to drive efficiency, reduce costs, and improve patient outcomes.
| Report Metric | Details |
| Report Name | e-clinical Trials Market |
| Accounted market size in year | US$ 609 million |
| Forecasted market size in 2031 | US$ 881 million |
| CAGR | 5.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Acceliant, ActiGraph, eClinicalWorks, IntrinsiQ Specialty Solutions, LMK Clinical Research Consulting, Lucidworks, Medrio, Parallel6, Symphony Clinical Research, Perceptive Informatics, EClinical Solutions, Ecrfplus, Clincase |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |