What is Global Pyrogen Testing Market?
The Global Pyrogen Testing Market is a crucial segment within the pharmaceutical and biotechnology industries, focusing on ensuring the safety and efficacy of medical products. Pyrogens are substances that can cause fever when they enter the human body, and they are typically found in bacterial endotoxins. The market for pyrogen testing is driven by the need to detect these fever-inducing substances in drugs, vaccines, and other medical products before they reach consumers. This testing is essential for maintaining product safety and compliance with regulatory standards. The market encompasses various testing methods, including the Limulus Amebocyte Lysate (LAL) test, which is widely used due to its sensitivity and reliability. As the demand for safe pharmaceutical products continues to rise, the pyrogen testing market is expected to grow, driven by advancements in testing technologies and increased regulatory scrutiny. The market's expansion is also fueled by the growing pharmaceutical and biotechnology sectors, which require rigorous testing to ensure product safety and efficacy. Overall, the Global Pyrogen Testing Market plays a vital role in safeguarding public health by preventing the distribution of contaminated medical products.
LAL Test, Chromogenic Test, Turbidimetric Test, Gel Clot Test, In Vitro Pyrogen Test, Rabbit Test in the Global Pyrogen Testing Market:
The Global Pyrogen Testing Market includes several key testing methods, each with its unique approach and application. The Limulus Amebocyte Lysate (LAL) test is one of the most commonly used methods for detecting bacterial endotoxins. It utilizes the blood of horseshoe crabs, which clots in the presence of endotoxins, making it a reliable and sensitive test. The LAL test is further divided into three types: the Chromogenic test, the Turbidimetric test, and the Gel Clot test. The Chromogenic test involves a color change reaction that occurs when endotoxins are present, allowing for quantitative measurement. The Turbidimetric test measures the cloudiness or turbidity that results from the reaction between endotoxins and the LAL reagent, providing a quantitative analysis. The Gel Clot test, on the other hand, is a qualitative test that detects the presence of endotoxins based on the formation of a gel clot. Each of these tests has its advantages and is chosen based on the specific requirements of the testing process. In addition to the LAL test, the In Vitro Pyrogen test is another method used in the market. This test involves the use of human blood cells to detect pyrogens, offering an alternative to animal-based testing methods. The Rabbit Test, although less commonly used today, involves injecting a sample into a rabbit and monitoring its temperature for any fever response. While the Rabbit Test has been largely replaced by more advanced methods, it remains a part of the historical context of pyrogen testing. The choice of testing method depends on various factors, including the type of product being tested, regulatory requirements, and the desired level of sensitivity and specificity. As the Global Pyrogen Testing Market continues to evolve, these testing methods are constantly being refined and improved to enhance their accuracy and reliability.
Pharmaceutical & Biotechnology Companies, Medical Devices, Other Applications in the Global Pyrogen Testing Market:
The Global Pyrogen Testing Market finds extensive usage across various sectors, including pharmaceutical and biotechnology companies, medical devices, and other applications. In pharmaceutical and biotechnology companies, pyrogen testing is a critical step in the development and manufacturing of drugs and vaccines. These companies rely on pyrogen testing to ensure that their products are free from harmful endotoxins that could cause adverse reactions in patients. The testing is conducted at various stages of the production process, from raw materials to finished products, to ensure compliance with regulatory standards and maintain product safety. In the medical devices sector, pyrogen testing is equally important. Medical devices that come into contact with the human body, such as surgical instruments, implants, and catheters, must be tested for pyrogens to prevent infections and complications. The testing helps manufacturers ensure that their devices are safe for use and meet the stringent requirements set by regulatory bodies. Beyond pharmaceuticals and medical devices, pyrogen testing is also used in other applications, such as the testing of water used in manufacturing processes, ensuring that it is free from endotoxins. This is particularly important in industries where water is a critical component of the production process, such as in the production of injectable drugs and vaccines. Overall, the Global Pyrogen Testing Market plays a vital role in ensuring the safety and efficacy of a wide range of products, protecting public health and maintaining consumer trust.
Global Pyrogen Testing Market Outlook:
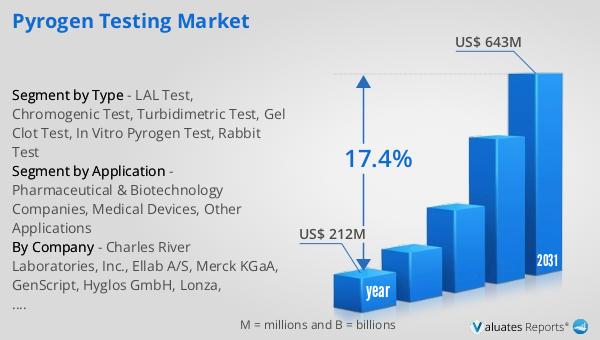
In 2024, the Global Pyrogen Testing Market was valued at approximately $212 million. This market is anticipated to experience significant growth, reaching an estimated value of $643 million by 2031. This growth trajectory represents a compound annual growth rate (CAGR) of 17.4% over the forecast period. The expansion of this market can be attributed to several factors, including the increasing demand for safe and effective pharmaceutical products, advancements in testing technologies, and heightened regulatory scrutiny. As pharmaceutical and biotechnology companies continue to innovate and develop new products, the need for rigorous pyrogen testing becomes more critical. Additionally, the growing awareness of the importance of product safety and the potential risks associated with endotoxin contamination are driving the demand for pyrogen testing services. The market's growth is also supported by the increasing adoption of advanced testing methods, such as the In Vitro Pyrogen test, which offers a more ethical and efficient alternative to traditional animal-based testing methods. As the Global Pyrogen Testing Market continues to evolve, it is expected to play an increasingly important role in ensuring the safety and efficacy of medical products, ultimately contributing to improved public health outcomes.
| Report Metric | Details |
| Report Name | Pyrogen Testing Market |
| Accounted market size in year | US$ 212 million |
| Forecasted market size in 2031 | US$ 643 million |
| CAGR | 17.4% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Charles River Laboratories, Inc., Ellab A/S, Merck KGaA, GenScript, Hyglos GmbH, Lonza, Associates of Cape Cod, Inc., Pyrostar |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |