What is Global Drug Safety Testing Market?
The Global Drug Safety Testing Market is a rapidly evolving sector that plays a crucial role in ensuring the safety and efficacy of pharmaceutical products. This market encompasses a wide range of services and technologies designed to evaluate the safety of drugs before they reach consumers. Drug safety testing involves various stages, including preclinical and clinical testing, to identify potential adverse effects and ensure compliance with regulatory standards. The market is driven by the increasing demand for safe and effective drugs, stringent regulatory requirements, and the growing prevalence of chronic diseases. Technological advancements, such as high-throughput screening and in vitro testing, have further propelled the market's growth by providing more efficient and accurate testing methods. As pharmaceutical companies strive to bring new drugs to market faster while ensuring safety, the demand for comprehensive drug safety testing services continues to rise. This market not only supports pharmaceutical companies but also biotechnology firms, medical device manufacturers, and other stakeholders involved in drug development and safety assessment. Overall, the Global Drug Safety Testing Market is a vital component of the healthcare industry, contributing to the development of safer and more effective therapeutic solutions.
Drug Testing, Drug Analysis in the Global Drug Safety Testing Market:
Drug testing and analysis are integral components of the Global Drug Safety Testing Market, ensuring that pharmaceutical products are safe for human use. Drug testing involves a series of scientific evaluations to determine the safety, efficacy, and quality of a drug. This process begins with preclinical testing, where drugs are tested in vitro (in the lab) and in vivo (in animals) to assess their biological activity and potential toxicity. These tests help identify any harmful effects before the drug is tested in humans. Once a drug passes preclinical testing, it enters clinical trials, which are conducted in multiple phases to evaluate the drug's safety and effectiveness in humans. Phase I trials focus on safety and dosage, Phase II trials assess efficacy and side effects, and Phase III trials confirm effectiveness and monitor adverse reactions in larger populations. Throughout these phases, drug analysis plays a critical role in ensuring the drug's chemical composition, stability, and purity. Advanced analytical techniques, such as chromatography and mass spectrometry, are used to detect impurities and ensure the drug meets quality standards. The data collected during drug testing and analysis are crucial for regulatory submissions and approvals. Regulatory agencies, such as the FDA and EMA, require comprehensive safety data to approve new drugs for market entry. In addition to traditional testing methods, the market has seen a rise in alternative testing approaches, such as in silico modeling and organ-on-chip technologies, which offer more ethical and cost-effective solutions. These innovations are particularly important in reducing the reliance on animal testing and improving the predictive accuracy of safety assessments. The Global Drug Safety Testing Market also addresses the challenges of drug interactions and adverse drug reactions, which can have significant implications for patient safety. By identifying potential interactions early in the drug development process, companies can mitigate risks and improve patient outcomes. Furthermore, post-marketing surveillance is an essential aspect of drug safety testing, as it monitors the long-term effects of drugs once they are available to the public. This ongoing evaluation helps identify rare adverse events and ensures that drugs remain safe throughout their lifecycle. Overall, drug testing and analysis are fundamental to the Global Drug Safety Testing Market, providing the necessary tools and data to develop safe and effective pharmaceutical products.
Pharmaceutical Company, Biotechnology Company, Medical Device Manufacturer, Other in the Global Drug Safety Testing Market:
The Global Drug Safety Testing Market serves a wide range of industries, including pharmaceutical companies, biotechnology firms, medical device manufacturers, and others involved in healthcare and drug development. Pharmaceutical companies are the primary users of drug safety testing services, as they are responsible for developing and bringing new drugs to market. These companies rely on comprehensive safety testing to ensure their products meet regulatory standards and are safe for human use. Drug safety testing helps pharmaceutical companies identify potential risks and adverse effects early in the development process, reducing the likelihood of costly recalls or litigation. Biotechnology companies also benefit from drug safety testing, as they often focus on developing innovative therapies and biologics. These companies require specialized testing services to evaluate the safety and efficacy of their products, which may involve complex biological processes and novel mechanisms of action. Drug safety testing provides the necessary data to support regulatory submissions and approvals, enabling biotechnology firms to bring their cutting-edge therapies to market. Medical device manufacturers are another key user of drug safety testing services, particularly when their products involve drug-device combinations. These manufacturers must ensure that their devices are safe and effective when used in conjunction with pharmaceutical products. Drug safety testing helps identify potential interactions and adverse effects, ensuring that the combined product meets regulatory requirements and provides optimal patient outcomes. Other industries, such as contract research organizations (CROs) and academic institutions, also utilize drug safety testing services to support their research and development efforts. CROs often provide outsourced testing services to pharmaceutical and biotechnology companies, offering expertise and resources to conduct comprehensive safety assessments. Academic institutions may engage in drug safety testing as part of their research initiatives, contributing to the development of new testing methods and technologies. Overall, the Global Drug Safety Testing Market plays a vital role in supporting the development and commercialization of safe and effective healthcare products across various industries. By providing essential testing services and data, this market helps ensure that new drugs and therapies meet the highest safety standards, ultimately benefiting patients and healthcare providers worldwide.
Global Drug Safety Testing Market Outlook:
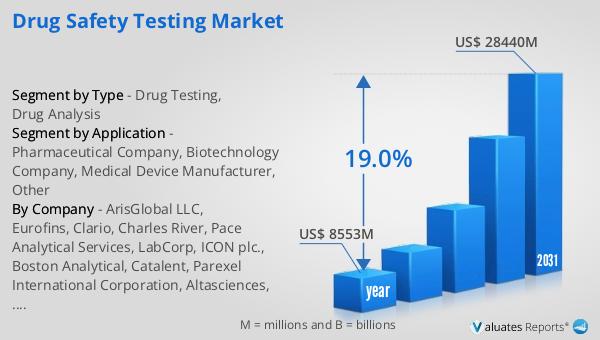
The global market for drug safety testing was valued at $8,553 million in 2024 and is anticipated to grow significantly, reaching an estimated size of $28,440 million by 2031, with a compound annual growth rate (CAGR) of 19.0% during this period. This growth reflects the increasing demand for drug safety testing services, which are essential for pharmaceutical companies and other stakeholders to conduct thorough drug component analysis and safety evaluations. The global pharmaceutical market, valued at $1,475 billion in 2022, is also experiencing growth, with a projected CAGR of 5% over the next six years. In comparison, the chemical drug market is expected to grow from $1,005 billion in 2018 to $1,094 billion by 2022. These figures highlight the expanding need for drug safety testing services as the pharmaceutical and chemical drug markets continue to evolve. The rising prevalence of chronic diseases, coupled with stringent regulatory requirements, drives the demand for comprehensive safety testing to ensure that new drugs are both safe and effective. As the market expands, technological advancements in testing methods, such as high-throughput screening and in vitro testing, are expected to further enhance the efficiency and accuracy of drug safety assessments. Overall, the Global Drug Safety Testing Market is poised for substantial growth, driven by the increasing need for safe and effective pharmaceutical products.
| Report Metric | Details |
| Report Name | Drug Safety Testing Market |
| Accounted market size in year | US$ 8553 million |
| Forecasted market size in 2031 | US$ 28440 million |
| CAGR | 19.0% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | ArisGlobal LLC, Eurofins, Clario, Charles River, Pace Analytical Services, LabCorp, ICON plc., Boston Analytical, Catalent, Parexel International Corporation, Altasciences, Hangzhou Huante Biotechnology Co., Ltd., Boende Testing, Microspectroscopy Technology Co., Ltd., Pharmaron, JOINN, Shanghai InnoStar Bio-tech Co., Ltd, WESTCHINA-FRONTIE PHARMA TECH, Shanghai Medicilon, Hangzhou Rongchuang Biological Co., Ltd., WuXi AppTec |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
