What is Global Cardiac Safety Assessment Services Market?
The Global Cardiac Safety Assessment Services Market is a specialized sector within the broader healthcare and pharmaceutical industries, focusing on evaluating the safety of cardiac functions during drug development and other medical interventions. This market plays a crucial role in ensuring that new drugs and treatments do not adversely affect the heart, which is vital for patient safety and regulatory approval. Cardiac safety assessments involve a range of tests and analyses, including electrocardiograms (ECGs), blood pressure monitoring, and other diagnostic tools to detect potential cardiac risks. These services are essential for pharmaceutical companies, research institutions, and healthcare providers as they navigate the complex landscape of drug development and patient care. The market is driven by the increasing prevalence of cardiovascular diseases, stringent regulatory requirements, and the growing demand for safe and effective therapeutics. As the healthcare industry continues to evolve, the Global Cardiac Safety Assessment Services Market is expected to expand, offering innovative solutions and technologies to meet the ever-changing needs of the medical community. This market not only ensures the safety of new drugs but also contributes to the overall advancement of healthcare by promoting the development of safer and more effective treatments.
Preclinical Cardiac Safety Assessment, Clinical Cardiac Safety Assessment in the Global Cardiac Safety Assessment Services Market:
Preclinical Cardiac Safety Assessment and Clinical Cardiac Safety Assessment are two critical components of the Global Cardiac Safety Assessment Services Market, each serving distinct yet interconnected roles in the drug development process. Preclinical cardiac safety assessment is the initial phase where potential cardiac risks of new drugs are evaluated before they are tested in humans. This phase involves a series of laboratory tests and animal studies designed to identify any adverse cardiac effects that a drug might have. Techniques such as in vitro assays, in vivo animal models, and computational modeling are commonly used to predict how a drug will interact with cardiac tissues. These assessments are crucial for identifying potential safety issues early in the development process, thereby reducing the risk of costly failures in later stages. On the other hand, Clinical Cardiac Safety Assessment takes place during the clinical trial phases, where the drug is tested on human subjects. This phase is more complex and involves continuous monitoring of cardiac functions through various diagnostic tools like ECGs, echocardiograms, and blood pressure measurements. The goal is to ensure that the drug is safe for human use and does not cause any harmful cardiac effects. Clinical assessments are conducted in multiple phases, starting with small groups of healthy volunteers and gradually expanding to larger populations, including patients with the target condition. Both preclinical and clinical assessments are governed by stringent regulatory guidelines to ensure the highest standards of safety and efficacy. The integration of advanced technologies such as artificial intelligence and machine learning is also transforming these assessments, enabling more accurate predictions and faster decision-making. As the demand for new and innovative drugs continues to grow, the importance of comprehensive cardiac safety assessments cannot be overstated. These assessments not only protect patients but also enhance the credibility and success of pharmaceutical companies by ensuring that only safe and effective drugs reach the market. In summary, preclinical and clinical cardiac safety assessments are indispensable components of the drug development process, providing critical insights into the safety profile of new therapeutics and ultimately contributing to the advancement of global healthcare.
Commissioned Research Institute, Pharmaceutical Company, Others in the Global Cardiac Safety Assessment Services Market:
The Global Cardiac Safety Assessment Services Market finds its application across various sectors, including Commissioned Research Institutes, Pharmaceutical Companies, and other entities involved in drug development and healthcare. Commissioned Research Institutes play a pivotal role in this market by conducting independent cardiac safety assessments for pharmaceutical companies and other clients. These institutes possess the expertise and resources to carry out comprehensive preclinical and clinical evaluations, providing unbiased and reliable data that is crucial for regulatory submissions and decision-making. By outsourcing cardiac safety assessments to these specialized institutes, pharmaceutical companies can focus on their core competencies while ensuring that their drug candidates meet the necessary safety standards. Pharmaceutical companies are the primary consumers of cardiac safety assessment services, as they are responsible for developing new drugs and bringing them to market. These companies rely heavily on both preclinical and clinical assessments to identify potential cardiac risks and ensure the safety of their products. The data obtained from these assessments is critical for gaining regulatory approval and minimizing the risk of adverse events once the drug is on the market. In addition to commissioned research institutes and pharmaceutical companies, other entities such as biotechnology firms, contract research organizations (CROs), and academic institutions also utilize cardiac safety assessment services. Biotechnology firms, often at the forefront of innovative drug development, require robust cardiac safety evaluations to support their cutting-edge research and development efforts. CROs, which provide outsourced research services to pharmaceutical and biotechnology companies, often include cardiac safety assessments as part of their comprehensive service offerings. Academic institutions, particularly those involved in translational research, also contribute to the cardiac safety assessment landscape by conducting studies that bridge the gap between basic research and clinical application. Overall, the Global Cardiac Safety Assessment Services Market is integral to the drug development ecosystem, providing essential services that ensure the safety and efficacy of new therapeutics. By catering to a diverse range of stakeholders, this market not only supports the development of safe and effective drugs but also fosters collaboration and innovation across the healthcare industry.
Global Cardiac Safety Assessment Services Market Outlook:
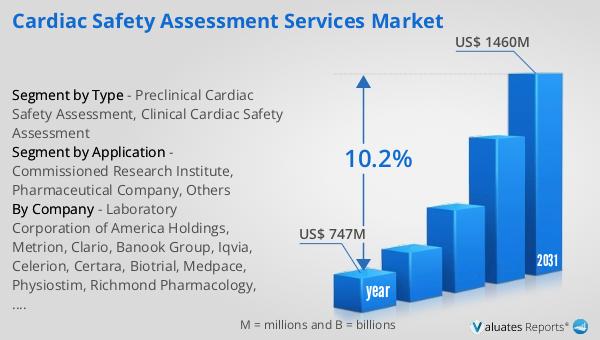
The global market for Cardiac Safety Assessment Services was valued at $747 million in 2024 and is anticipated to grow significantly, reaching an estimated size of $1,460 million by 2031. This growth trajectory represents a compound annual growth rate (CAGR) of 10.2% over the forecast period. This robust expansion is indicative of the increasing demand for cardiac safety assessments, driven by the rising prevalence of cardiovascular diseases and the need for stringent safety evaluations in drug development. As pharmaceutical companies and research institutions continue to innovate and develop new therapeutics, the importance of comprehensive cardiac safety assessments becomes even more pronounced. These services are essential for ensuring that new drugs do not pose undue risks to patients, thereby facilitating regulatory approval and market success. The projected growth of the Global Cardiac Safety Assessment Services Market underscores the critical role these services play in the healthcare industry, providing the necessary tools and expertise to navigate the complex landscape of drug development and patient safety. As the market continues to evolve, stakeholders can expect to see further advancements in technology and methodology, enhancing the accuracy and efficiency of cardiac safety assessments and ultimately contributing to the advancement of global healthcare.
| Report Metric | Details |
| Report Name | Cardiac Safety Assessment Services Market |
| Accounted market size in year | US$ 747 million |
| Forecasted market size in 2031 | US$ 1460 million |
| CAGR | 10.2% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Laboratory Corporation of America Holdings, Metrion, Clario, Banook Group, Iqvia, Celerion, Certara, Biotrial, Medpace, Physiostim, Richmond Pharmacology, Ncardia, Pharmaceutical Product Development Llc., Reaction Biology, Eurofins Discovery, Altasciences, NEXEL, charles river, Scottish Institute of Electrophysiology, Ronovation Biotech, Elixir Clinical Research |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
