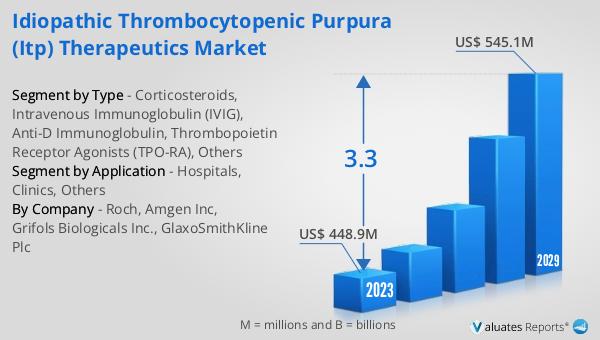
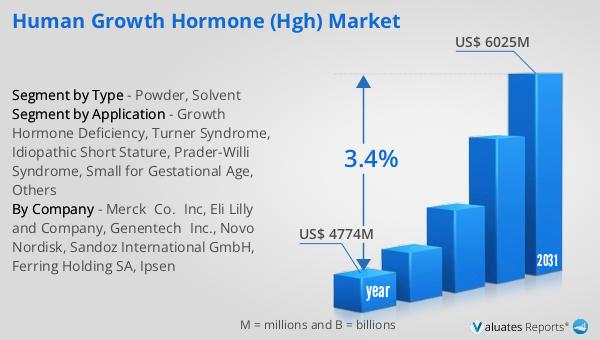
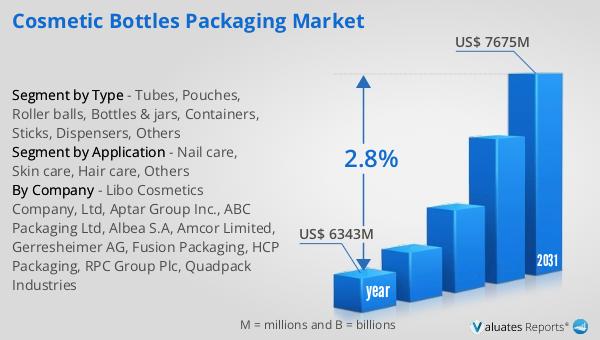
What is Global Herbal Cosmetic Market?
The Global Herbal Cosmetic Market is a rapidly expanding sector within the beauty and personal care industry, driven by increasing consumer awareness and preference for natural and organic products. Herbal cosmetics are made from plant-derived ingredients, which are perceived as safer and more environmentally friendly compared to synthetic alternatives. This market encompasses a wide range of products, including skincare, haircare, and makeup, all formulated with botanical extracts and essential oils. The demand for herbal cosmetics is fueled by a growing trend towards holistic wellness and sustainable living, as consumers become more conscious of the ingredients in their personal care products. Additionally, the rise in disposable income and changing lifestyles, particularly in emerging economies, are contributing to the market's growth. The global herbal cosmetic market is characterized by a diverse range of products catering to various skin types and concerns, offering consumers a plethora of choices. As more people seek to incorporate natural elements into their beauty routines, the market is poised for continued expansion, with companies investing in research and development to innovate and meet the evolving needs of consumers.

For Men, For Women in the Global Herbal Cosmetic Market:
In the Global Herbal Cosmetic Market, products are often tailored to meet the specific needs of different genders, with distinct offerings for men and women. For men, the market has seen a significant rise in demand for herbal grooming products, as more men become conscious of their appearance and personal care. Herbal products for men typically include beard oils, aftershaves, shampoos, and skincare items that are formulated to address common male concerns such as skin sensitivity, acne, and hair loss. These products often contain ingredients like tea tree oil, aloe vera, and eucalyptus, known for their soothing and healing properties. The emphasis is on providing effective solutions that are gentle on the skin, catering to the growing trend of men seeking natural alternatives to chemical-laden products. On the other hand, the market for women is more expansive, with a wide array of herbal cosmetics available to address various beauty needs. Women are increasingly opting for herbal skincare products like cleansers, moisturizers, and serums that are free from harsh chemicals and artificial fragrances. Ingredients such as chamomile, lavender, and rosehip oil are popular for their anti-aging and rejuvenating properties. Herbal haircare products for women, including shampoos, conditioners, and hair masks, are also in high demand, as they promise to nourish and strengthen hair without the use of sulfates and parabens. Makeup products, such as foundations, lipsticks, and eyeshadows, are being reformulated with natural pigments and plant-based ingredients to appeal to eco-conscious consumers. The focus is on enhancing beauty while maintaining skin health, with an emphasis on transparency and sustainability in product formulations. As the herbal cosmetic market continues to grow, both men and women are benefiting from a wider selection of products that align with their values and lifestyle choices, promoting a more natural approach to beauty and personal care.
Cleaning, Anti Disease in the Global Herbal Cosmetic Market:
The usage of herbal cosmetics in the areas of cleaning and anti-disease is gaining traction as consumers seek products that offer both efficacy and safety. In terms of cleaning, herbal cosmetics provide gentle yet effective cleansing solutions for the skin and hair. Herbal cleansers, such as face washes and body soaps, are formulated with natural ingredients like neem, turmeric, and green tea, which are known for their antibacterial and antioxidant properties. These ingredients help to cleanse the skin without stripping it of its natural oils, making them suitable for all skin types, including sensitive skin. Herbal shampoos and conditioners are also popular for their ability to cleanse the scalp and hair while promoting overall hair health. Ingredients like hibiscus, amla, and shikakai are commonly used for their nourishing and strengthening benefits, helping to maintain a healthy scalp and prevent hair damage. In the realm of anti-disease, herbal cosmetics are increasingly being recognized for their potential to support skin health and prevent common skin issues. Products containing ingredients such as aloe vera, calendula, and tea tree oil are valued for their anti-inflammatory and antimicrobial properties, which can help soothe irritated skin and reduce the occurrence of acne and other skin conditions. Herbal formulations are also being explored for their potential to address more serious skin concerns, such as eczema and psoriasis, offering a natural alternative to conventional treatments. The emphasis on using plant-based ingredients not only aligns with consumer preferences for natural products but also highlights the potential therapeutic benefits of herbs in promoting skin health and preventing disease. As research continues to uncover the benefits of herbal ingredients, the global herbal cosmetic market is poised to expand its offerings in the areas of cleaning and anti-disease, providing consumers with safe and effective options for their personal care needs.
Global Herbal Cosmetic Market Outlook:
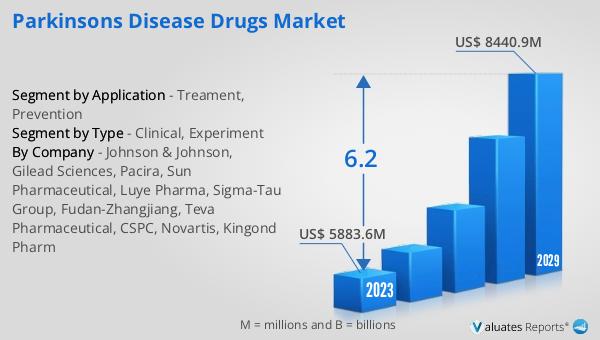
The global pharmaceutical market was valued at approximately 1,475 billion USD in 2022, with projections indicating a steady growth rate of 5% annually over the next six years. This growth is indicative of the increasing demand for pharmaceutical products worldwide, driven by factors such as an aging population, rising prevalence of chronic diseases, and advancements in medical technology. In comparison, the chemical drug market has also shown significant growth, with its value rising from 1,005 billion USD in 2018 to an estimated 1,094 billion USD in 2022. This increase reflects the ongoing demand for chemical-based medications, which continue to play a crucial role in the treatment and management of various health conditions. The growth in both the pharmaceutical and chemical drug markets underscores the importance of continued innovation and investment in the healthcare sector to meet the evolving needs of patients and healthcare providers. As the global population continues to grow and age, the demand for effective and accessible healthcare solutions is expected to drive further expansion in these markets, highlighting the critical role they play in improving health outcomes and quality of life for individuals around the world.
| Report Metric | Details |
| Report Name | Herbal Cosmetic Market |
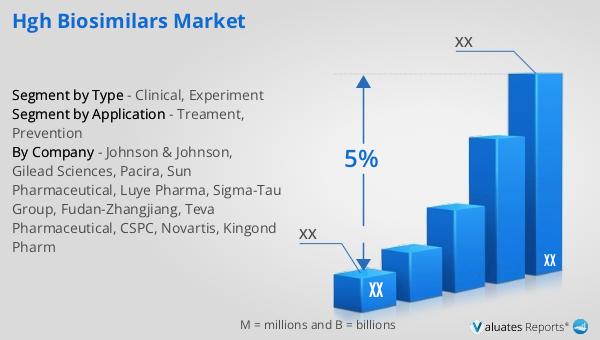
| CAGR | 5% |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | L'Oral, P&G, Shiseido, Unilever, Beiersdorf, Amway, AVON Beauty Products, Burberry, INVERSIONES AVI AMERICA, Chanel, Clarins |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |