What is Global Preclinical CRO Treatment Market?
The Global Preclinical CRO Treatment Market is a vital segment within the pharmaceutical and biotechnology industries, focusing on the early stages of drug development. Preclinical Contract Research Organizations (CROs) provide essential services that help in the evaluation of potential drug candidates before they proceed to clinical trials. These services include a wide range of laboratory tests and studies that assess the safety, efficacy, and pharmacokinetics of new compounds. The market is driven by the increasing demand for outsourcing research and development activities, as it allows companies to reduce costs and focus on their core competencies. Additionally, the rise in chronic diseases and the need for innovative therapies have further fueled the growth of this market. Preclinical CROs play a crucial role in ensuring that new drugs are safe and effective, thereby accelerating the drug development process and bringing new treatments to market more efficiently. The global reach of these organizations enables them to offer diverse expertise and advanced technologies, making them indispensable partners in the pharmaceutical industry.
Bioanalysis and DMPK Studies, Toxicology Testing in the Global Preclinical CRO Treatment Market:
Bioanalysis and DMPK (Drug Metabolism and Pharmacokinetics) studies are integral components of the Global Preclinical CRO Treatment Market. These studies are crucial for understanding the absorption, distribution, metabolism, and excretion (ADME) of drug candidates. Bioanalysis involves the quantitative measurement of drugs and their metabolites in biological systems, which is essential for determining the pharmacokinetic profile of a compound. This information helps in optimizing dosing regimens and ensuring that the drug reaches its target site in the body at therapeutic concentrations. DMPK studies, on the other hand, provide insights into how a drug is processed within the body, which is vital for predicting its behavior in humans. These studies help in identifying potential drug-drug interactions and assessing the impact of genetic variations on drug metabolism. Toxicology testing is another critical aspect of preclinical research, aimed at evaluating the safety of new drug candidates. It involves a series of tests to determine the potential adverse effects of a compound on various biological systems. These tests are conducted in vitro (in a controlled environment outside a living organism) and in vivo (within a living organism) to assess the compound's toxicity profile. The data obtained from toxicology studies are used to establish safe starting doses for clinical trials and to identify any potential risks associated with the drug. The integration of bioanalysis, DMPK, and toxicology testing in preclinical research ensures a comprehensive evaluation of drug candidates, thereby reducing the likelihood of failures in later stages of development. The Global Preclinical CRO Treatment Market is characterized by the presence of specialized service providers that offer state-of-the-art facilities and expertise in these areas. These organizations employ advanced technologies and methodologies to deliver accurate and reliable data, which is crucial for making informed decisions during drug development. The demand for bioanalysis and DMPK studies, along with toxicology testing, is expected to grow as pharmaceutical companies continue to seek efficient and cost-effective solutions for their preclinical research needs. By outsourcing these services to CROs, companies can leverage the expertise and resources of these organizations, allowing them to focus on their core competencies and accelerate the development of new therapies. The collaboration between pharmaceutical companies and preclinical CROs is essential for advancing drug discovery and development, ultimately leading to the introduction of innovative treatments that address unmet medical needs.
Biopharmaceutical Companies, Government and Academic Institutes, Medical Device Companies in the Global Preclinical CRO Treatment Market:
The Global Preclinical CRO Treatment Market plays a significant role in supporting various sectors, including biopharmaceutical companies, government and academic institutes, and medical device companies. Biopharmaceutical companies rely heavily on preclinical CROs to conduct essential research and testing during the early stages of drug development. By outsourcing these activities, companies can reduce costs, access specialized expertise, and accelerate the development process. Preclinical CROs provide a range of services, including bioanalysis, DMPK studies, and toxicology testing, which are crucial for evaluating the safety and efficacy of new drug candidates. This collaboration allows biopharmaceutical companies to focus on their core competencies, such as drug discovery and commercialization, while ensuring that their products meet regulatory requirements. Government and academic institutes also benefit from the services offered by preclinical CROs. These organizations often engage in research projects aimed at advancing scientific knowledge and developing new therapies for various diseases. Preclinical CROs provide the necessary infrastructure and expertise to support these initiatives, enabling researchers to conduct high-quality studies and generate reliable data. By partnering with CROs, government and academic institutes can leverage advanced technologies and methodologies, which are essential for conducting cutting-edge research. This collaboration fosters innovation and contributes to the development of new treatments that address unmet medical needs. Medical device companies also utilize the services of preclinical CROs to evaluate the safety and performance of their products. Preclinical testing is essential for assessing the biocompatibility and functionality of medical devices before they are introduced to the market. CROs offer a range of testing services, including in vitro and in vivo studies, to ensure that medical devices meet regulatory standards and are safe for use in humans. By outsourcing these activities to CROs, medical device companies can access specialized expertise and resources, allowing them to focus on product development and commercialization. The Global Preclinical CRO Treatment Market is characterized by the presence of specialized service providers that offer state-of-the-art facilities and expertise in various areas of preclinical research. These organizations employ advanced technologies and methodologies to deliver accurate and reliable data, which is crucial for making informed decisions during product development. The demand for preclinical CRO services is expected to grow as companies continue to seek efficient and cost-effective solutions for their research needs. By partnering with CROs, biopharmaceutical companies, government and academic institutes, and medical device companies can accelerate the development of new therapies and products, ultimately leading to improved patient outcomes and advancements in healthcare.
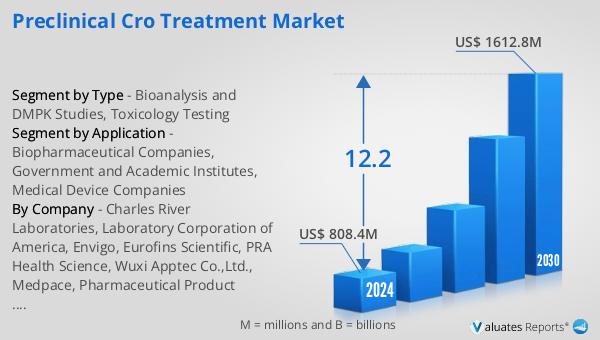
Global Preclinical CRO Treatment Market Outlook:
The global market for Preclinical CRO Treatment was valued at $897 million in 2024 and is anticipated to expand significantly, reaching an estimated $1,986 million by 2031. This growth trajectory reflects a compound annual growth rate (CAGR) of 12.2% over the forecast period. This robust expansion underscores the increasing reliance on preclinical CROs by pharmaceutical and biotechnology companies to streamline their drug development processes. The market's growth is driven by several factors, including the rising demand for outsourcing research and development activities, the need for cost-effective solutions, and the growing complexity of drug discovery and development. As companies strive to bring innovative therapies to market more efficiently, preclinical CROs play a crucial role in providing the necessary expertise and resources. The projected growth of the Global Preclinical CRO Treatment Market highlights the importance of these organizations in advancing drug discovery and development, ultimately leading to the introduction of new treatments that address unmet medical needs. The market's expansion also reflects the increasing collaboration between pharmaceutical companies and CROs, as they work together to accelerate the development of new therapies and improve patient outcomes. This partnership is essential for driving innovation and ensuring that new drugs are safe and effective, thereby contributing to the overall advancement of healthcare.
| Report Metric | Details |
| Report Name | Preclinical CRO Treatment Market |
| Accounted market size in year | US$ 897 million |
| Forecasted market size in 2031 | US$ 1986 million |
| CAGR | 12.2% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type | |
| Segment by Application |
|
| By Region |
|
| By Company | Charles River Laboratories, Laboratory Corporation of America, Envigo, Eurofins Scientific, PRA Health Science, Wuxi Apptec Co.,Ltd., Medpace, Pharmaceutical Product Development, Paraxel, Pharmaron, Joinn Laboratories, Medicilon Inc., Crown Bioscience, Yinuosi Bio-Technology |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
