What is Global Cefalexin API Market?
The Global Cefalexin API Market refers to the worldwide industry focused on the production and distribution of Cefalexin Active Pharmaceutical Ingredient (API). Cefalexin is a widely used antibiotic belonging to the cephalosporin class, which is effective against a variety of bacterial infections. The API is the core component used in the formulation of Cefalexin-based medications. This market encompasses the entire supply chain, from raw material procurement to the manufacturing processes that yield the final API product. The demand for Cefalexin API is driven by its extensive use in treating infections in both humans and animals, making it a crucial component in the pharmaceutical industry. The market is characterized by a competitive landscape with numerous manufacturers and suppliers striving to meet the global demand while adhering to stringent regulatory standards. As healthcare needs continue to evolve, the Global Cefalexin API Market plays a vital role in ensuring the availability of effective antibiotic treatments worldwide.
>99.5, >99.8 in the Global Cefalexin API Market:
In the Global Cefalexin API Market, the purity levels of the API are critical, with specifications often exceeding 99.5% and 99.8%. These purity levels are essential for ensuring the efficacy and safety of the antibiotic when used in medical treatments. A purity level of >99.5% indicates that the API contains less than 0.5% impurities, which is crucial for maintaining the drug's effectiveness and minimizing potential side effects. Similarly, a purity level of >99.8% signifies an even higher standard, with impurities constituting less than 0.2% of the total composition. Achieving such high purity levels requires advanced manufacturing processes and stringent quality control measures. Manufacturers employ sophisticated techniques such as crystallization, filtration, and chromatography to isolate and purify the Cefalexin API to the desired specifications. These processes are meticulously monitored to ensure consistency and compliance with international pharmaceutical standards. The choice between >99.5% and >99.8% purity levels often depends on the specific requirements of pharmaceutical companies and the intended application of the antibiotic. Higher purity levels are typically preferred for formulations that demand exceptional quality and performance, such as injectable forms of the medication. The emphasis on purity is not only a regulatory requirement but also a competitive differentiator in the market, as pharmaceutical companies seek to offer the most reliable and effective products to healthcare providers and patients. As a result, the Global Cefalexin API Market is characterized by continuous innovation and investment in research and development to enhance production techniques and achieve superior purity standards. This focus on quality assurance ensures that the Cefalexin API meets the rigorous demands of the global healthcare industry, providing a reliable foundation for the development of life-saving antibiotics.
Powder Injection, Injection in the Global Cefalexin API Market:
The Global Cefalexin API Market finds significant application in the production of powder injections and injectable solutions, which are critical forms of antibiotic administration. Powder injections involve the use of Cefalexin API in a lyophilized or freeze-dried form, which is reconstituted with a suitable solvent before administration. This form is particularly advantageous for its stability and extended shelf life, making it ideal for storage and transportation. The reconstitution process allows healthcare providers to prepare the injection immediately before use, ensuring the potency and effectiveness of the antibiotic. Injectable solutions, on the other hand, involve the direct use of Cefalexin API in a liquid form, ready for administration. This form is preferred for its convenience and ease of use, especially in clinical settings where rapid treatment is necessary. The use of Cefalexin API in these injectable forms is crucial for treating severe bacterial infections that require immediate intervention. The rapid onset of action provided by injections ensures that the antibiotic reaches the bloodstream quickly, delivering therapeutic effects more efficiently than oral medications. This is particularly important in hospital settings where patients may be critically ill and require prompt treatment. The production of Cefalexin API for injections involves stringent quality control measures to ensure sterility and safety. Manufacturers must adhere to rigorous standards to prevent contamination and ensure that the final product is free from impurities. This involves advanced manufacturing techniques and thorough testing at various stages of production. The demand for Cefalexin API in powder and injectable forms is driven by the need for effective and reliable antibiotic treatments in both human and veterinary medicine. As bacterial resistance continues to pose a challenge to global health, the role of Cefalexin API in providing potent and versatile treatment options remains indispensable. The Global Cefalexin API Market, therefore, plays a pivotal role in supporting healthcare systems worldwide by ensuring the availability of high-quality antibiotics in injectable forms.
Global Cefalexin API Market Outlook:
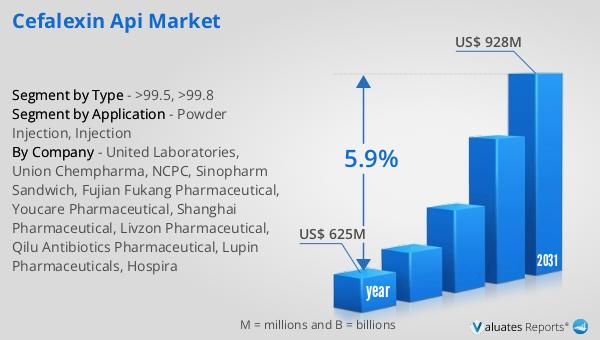
The global market for Cefalexin API was valued at $625 million in 2024 and is anticipated to grow to a revised size of $928 million by 2031, reflecting a compound annual growth rate (CAGR) of 5.9% during the forecast period. This growth trajectory underscores the increasing demand for Cefalexin API, driven by its widespread use in treating bacterial infections. In comparison, the global pharmaceutical market was valued at $1,475 billion in 2022, with a projected CAGR of 5% over the next six years. This indicates a robust expansion of the pharmaceutical sector, with Cefalexin API playing a significant role within this landscape. Meanwhile, the chemical drug market, which includes a broad range of pharmaceutical products, was estimated to grow from $1,005 billion in 2018 to $1,094 billion in 2022. This growth highlights the dynamic nature of the pharmaceutical industry and the critical role of APIs like Cefalexin in meeting the global demand for effective medications. The Cefalexin API market's growth is indicative of the broader trends in the pharmaceutical industry, where innovation, quality, and efficacy are paramount. As healthcare needs continue to evolve, the market for Cefalexin API is poised to play a crucial role in addressing the challenges of bacterial infections and supporting the development of new and improved antibiotic therapies.
| Report Metric | Details |
| Report Name | Cefalexin API Market |
| Accounted market size in year | US$ 625 million |
| Forecasted market size in 2031 | US$ 928 million |
| CAGR | 5.9% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | United Laboratories, Union Chempharma, NCPC, Sinopharm Sandwich, Fujian Fukang Pharmaceutical, Youcare Pharmaceutical, Shanghai Pharmaceutical, Livzon Pharmaceutical, Qilu Antibiotics Pharmaceutical, Lupin Pharmaceuticals, Hospira |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |