What is Global Beraprost Sodium Market?
The Global Beraprost Sodium Market is a specialized segment within the pharmaceutical industry, focusing on the production and distribution of Beraprost Sodium, a synthetic analog of prostacyclin. This compound is primarily used in the treatment of pulmonary arterial hypertension (PAH), a condition characterized by high blood pressure in the arteries of the lungs. Beraprost Sodium works by dilating blood vessels and inhibiting platelet aggregation, which helps to reduce blood pressure and improve blood flow. The market for this compound is driven by the increasing prevalence of PAH, advancements in drug formulation, and a growing awareness of the condition. Additionally, the market is influenced by regulatory approvals, patent expirations, and competitive dynamics among pharmaceutical companies. As healthcare systems worldwide continue to evolve, the demand for effective treatments like Beraprost Sodium is expected to grow, making this market an important area of focus for stakeholders in the pharmaceutical industry. The market's growth is also supported by ongoing research and development efforts aimed at improving the efficacy and safety profile of Beraprost Sodium, as well as exploring its potential applications in other therapeutic areas.
Tablet, Sustained Release Tablets in the Global Beraprost Sodium Market:
In the Global Beraprost Sodium Market, tablets and sustained release tablets play a crucial role in the administration of this medication. Tablets are the most common form of Beraprost Sodium, offering a convenient and straightforward method for patients to manage their condition. These tablets are typically taken orally, allowing for easy absorption into the bloodstream. The standard tablet formulation is designed to provide a quick onset of action, which is essential for patients who require immediate relief from symptoms associated with pulmonary arterial hypertension. However, one of the challenges with standard tablets is the need for multiple daily doses to maintain therapeutic levels of the drug in the body. This can be inconvenient for patients and may lead to issues with adherence to the prescribed treatment regimen. To address this challenge, sustained release tablets have been developed. These tablets are formulated to release Beraprost Sodium gradually over an extended period, allowing for a more consistent therapeutic effect with fewer doses. Sustained release tablets offer several advantages, including improved patient compliance, reduced frequency of dosing, and potentially enhanced efficacy due to more stable drug levels in the bloodstream. The development of sustained release formulations is a significant advancement in the Global Beraprost Sodium Market, as it aligns with the broader trend in the pharmaceutical industry towards improving patient convenience and treatment outcomes. The production of these tablets involves sophisticated manufacturing processes to ensure the controlled release of the active ingredient. This requires a deep understanding of the drug's pharmacokinetics and the use of advanced technologies to create the desired release profile. The sustained release tablets are particularly beneficial for patients who have difficulty adhering to a multiple-dose regimen, as they simplify the treatment process and reduce the burden of frequent medication intake. Furthermore, sustained release formulations may also help to minimize side effects associated with peak drug concentrations, as they provide a more even distribution of the drug over time. This can lead to a better overall treatment experience for patients and may improve long-term outcomes. The availability of both standard and sustained release tablets in the Global Beraprost Sodium Market provides healthcare providers with options to tailor treatment plans to the individual needs of patients. This flexibility is important, as it allows for personalized approaches to managing pulmonary arterial hypertension, taking into account factors such as the severity of the condition, patient lifestyle, and potential interactions with other medications. As the market continues to evolve, ongoing research and development efforts are likely to focus on further optimizing these formulations to enhance their therapeutic benefits and address any remaining challenges. The Global Beraprost Sodium Market is poised for growth as these advancements continue to improve the quality of life for patients with pulmonary arterial hypertension.
Hospital, Clinic in the Global Beraprost Sodium Market:
The usage of Beraprost Sodium in hospitals and clinics is a critical aspect of the Global Beraprost Sodium Market. In hospital settings, Beraprost Sodium is often used as part of a comprehensive treatment plan for patients with pulmonary arterial hypertension (PAH). Hospitals provide an environment where patients can receive a multidisciplinary approach to their care, involving cardiologists, pulmonologists, and other specialists. This collaborative approach ensures that patients receive the most effective treatment strategies, which may include Beraprost Sodium as a key component. In hospitals, the administration of Beraprost Sodium is closely monitored by healthcare professionals to ensure optimal dosing and to manage any potential side effects. This is particularly important for patients with severe PAH, who may require more intensive management and monitoring. The use of Beraprost Sodium in hospitals also allows for the integration of other therapeutic interventions, such as oxygen therapy and lifestyle modifications, to enhance treatment outcomes. In clinics, Beraprost Sodium is typically prescribed for outpatient management of PAH. Clinics offer a more accessible setting for patients to receive ongoing care and follow-up. In this context, Beraprost Sodium is often part of a long-term management plan aimed at controlling symptoms and preventing disease progression. The convenience of clinic visits allows patients to maintain regular contact with their healthcare providers, ensuring that their treatment plan is adjusted as needed based on their response to therapy. Clinics also play a vital role in patient education, providing information on the proper use of Beraprost Sodium, potential side effects, and the importance of adherence to the prescribed regimen. This educational component is crucial, as it empowers patients to take an active role in managing their condition and can lead to improved treatment adherence and outcomes. The use of Beraprost Sodium in both hospitals and clinics highlights the importance of a coordinated approach to managing pulmonary arterial hypertension. By providing access to this medication in different healthcare settings, patients can benefit from a continuum of care that addresses their needs at various stages of their treatment journey. The integration of Beraprost Sodium into hospital and clinic protocols reflects its established role in the management of PAH and underscores the need for ongoing collaboration between healthcare providers to optimize patient outcomes. As the Global Beraprost Sodium Market continues to evolve, the focus on improving access to this medication in both hospital and clinic settings will remain a priority, ensuring that patients with PAH receive the best possible care.
Global Beraprost Sodium Market Outlook:
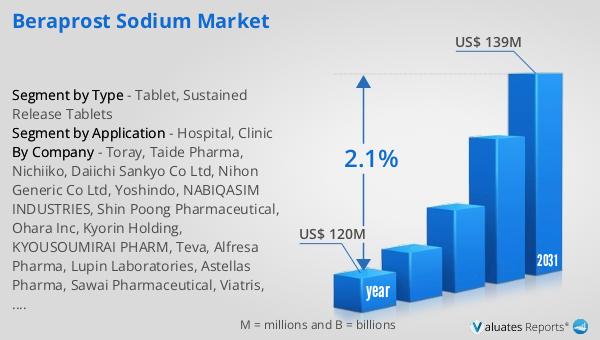
In 2024, the global market for Beraprost Sodium was valued at $120 million. By 2031, it is anticipated to grow to a revised size of $139 million, reflecting a compound annual growth rate (CAGR) of 2.1% over the forecast period. This growth trajectory indicates a steady increase in demand for Beraprost Sodium, driven by factors such as the rising prevalence of pulmonary arterial hypertension and advancements in drug formulations. The market's expansion is also supported by increased awareness of the condition and the availability of improved treatment options. As healthcare systems worldwide continue to prioritize the management of chronic conditions, the demand for effective therapies like Beraprost Sodium is expected to rise. The projected growth in the market size underscores the importance of ongoing research and development efforts to enhance the efficacy and safety profile of Beraprost Sodium. Additionally, the market dynamics are influenced by regulatory approvals, patent expirations, and competitive pressures among pharmaceutical companies. As the market evolves, stakeholders will need to navigate these challenges while capitalizing on opportunities for growth. The steady growth rate of the Beraprost Sodium market reflects its established role in the treatment of pulmonary arterial hypertension and highlights the need for continued innovation to meet the evolving needs of patients and healthcare providers.
| Report Metric | Details |
| Report Name | Beraprost Sodium Market |
| Accounted market size in year | US$ 120 million |
| Forecasted market size in 2031 | US$ 139 million |
| CAGR | 2.1% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Toray, Taide Pharma, Nichiiko, Daiichi Sankyo Co Ltd, Nihon Generic Co Ltd, Yoshindo, NABIQASIM INDUSTRIES, Shin Poong Pharmaceutical, Ohara Inc, Kyorin Holding, KYOUSOUMIRAI PHARM, Teva, Alfresa Pharma, Lupin Laboratories, Astellas Pharma, Sawai Pharmaceutical, Viatris, Kaken Pharmaceutical |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |