What is Global Inactivated Polio Virus Vaccine Market?
The Global Inactivated Polio Virus Vaccine Market is a crucial segment within the pharmaceutical industry, focusing on the development and distribution of vaccines designed to prevent polio, a highly infectious viral disease. Polio primarily affects children and can lead to permanent paralysis or even death. The inactivated polio vaccine (IPV) is a key tool in the global effort to eradicate polio, as it contains an inactivated virus that cannot cause the disease. This vaccine is administered via injection and is considered safe and effective, providing immunity against all three types of poliovirus. The market for IPV is driven by the ongoing need to maintain high immunization coverage to prevent outbreaks, especially in regions where polio is still endemic or where there is a risk of reintroduction. Additionally, the market is influenced by government initiatives, international health organizations, and partnerships aimed at increasing vaccine accessibility and affordability. The demand for IPV is also supported by the global push towards achieving the World Health Organization's goal of a polio-free world. As such, the Global Inactivated Polio Virus Vaccine Market plays a vital role in public health, contributing to the prevention of a debilitating disease and supporting global health security.
Type I, Type II, Type III in the Global Inactivated Polio Virus Vaccine Market:
The Global Inactivated Polio Virus Vaccine Market is categorized into three main types based on the strains of poliovirus they target: Type I, Type II, and Type III. Each type corresponds to a different strain of the poliovirus, and the vaccines are designed to provide immunity against these specific strains. Type I poliovirus is the most common and historically has been responsible for the majority of polio cases worldwide. Vaccines targeting Type I are crucial in preventing outbreaks, especially in areas where this strain is prevalent. Type II poliovirus, although declared eradicated in the wild in 2015, still poses a risk due to vaccine-derived strains that can circulate in under-immunized populations. Vaccines that include Type II components are essential in maintaining immunity and preventing the re-emergence of this strain. Type III poliovirus is less common but still a significant concern, as it can cause outbreaks in areas with low vaccination coverage. The inclusion of Type III in vaccines ensures comprehensive protection against all poliovirus strains. The development and distribution of these vaccines are influenced by various factors, including epidemiological data, regional polio prevalence, and global health initiatives. Manufacturers and health organizations work together to ensure that vaccines are available and accessible to populations at risk. The production of these vaccines involves complex processes, including the cultivation of the virus, inactivation, and formulation into a safe and effective vaccine. Quality control and regulatory compliance are critical to ensure the safety and efficacy of the vaccines. The market for these vaccines is also shaped by technological advancements, such as the development of combination vaccines that include IPV along with other antigens, providing broader protection and simplifying immunization schedules. Additionally, the market is influenced by the transition from oral polio vaccines (OPV) to IPV, driven by the need to eliminate the risk of vaccine-derived poliovirus associated with OPV. This transition requires significant investment in infrastructure, training, and public awareness campaigns to ensure successful implementation. The Global Inactivated Polio Virus Vaccine Market is a dynamic and evolving sector, responding to changes in polio epidemiology, technological advancements, and global health priorities. The collaboration between governments, international organizations, and the private sector is essential to ensure the continued availability and accessibility of these life-saving vaccines. As the world moves closer to achieving polio eradication, the role of IPV in maintaining immunity and preventing outbreaks remains critical. The market's growth is supported by ongoing research and development efforts aimed at improving vaccine formulations, delivery methods, and distribution systems. These efforts are crucial in addressing the challenges of reaching remote and underserved populations, ensuring that no child is left vulnerable to polio. The Global Inactivated Polio Virus Vaccine Market is a testament to the power of vaccines in preventing disease and protecting public health.
Hospital, Clinic in the Global Inactivated Polio Virus Vaccine Market:
The usage of the Global Inactivated Polio Virus Vaccine Market in hospitals and clinics is integral to the broader public health strategy aimed at eradicating polio. Hospitals serve as primary centers for administering the inactivated polio vaccine (IPV), especially in urban and densely populated areas. They are equipped with the necessary infrastructure and trained healthcare professionals to ensure the safe and effective delivery of vaccines. In hospitals, IPV is often administered as part of routine immunization programs for infants and children, ensuring that they receive the vaccine at the recommended ages. This setting also allows for the monitoring of vaccine efficacy and the management of any adverse reactions, providing a controlled environment for vaccination. Clinics, on the other hand, play a crucial role in extending the reach of IPV to rural and underserved communities. They often serve as the first point of contact for healthcare in these areas, making them vital in ensuring that all children have access to life-saving vaccines. Clinics may operate as standalone facilities or as part of larger healthcare networks, and they often collaborate with government health departments and non-governmental organizations to conduct vaccination campaigns. These campaigns are essential in maintaining high immunization coverage and preventing polio outbreaks. The role of clinics is particularly important in regions where healthcare infrastructure is limited, as they provide a more accessible and affordable option for vaccination. Both hospitals and clinics are supported by national and international health organizations, which provide funding, technical assistance, and logistical support to ensure the availability and distribution of IPV. This support is crucial in overcoming challenges such as vaccine shortages, cold chain management, and public awareness. The collaboration between these healthcare facilities and global health initiatives is essential in achieving the goal of a polio-free world. The usage of IPV in hospitals and clinics is also influenced by policy decisions and regulatory frameworks, which dictate the inclusion of IPV in national immunization schedules and the allocation of resources for vaccination programs. These policies are informed by epidemiological data, cost-effectiveness analyses, and the recommendations of expert advisory groups. The integration of IPV into routine healthcare services in hospitals and clinics is a testament to the commitment of the global health community to eradicate polio and protect future generations from this debilitating disease. The continued success of these efforts depends on sustained investment in healthcare infrastructure, workforce training, and public education to ensure that all children receive the full benefits of vaccination.
Global Inactivated Polio Virus Vaccine Market Outlook:
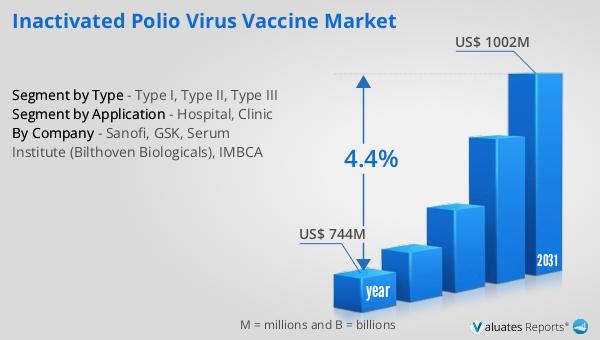
In 2024, the global market for Inactivated Polio Virus Vaccine was valued at approximately $744 million. By 2031, it is anticipated to grow to a revised size of around $1,002 million, reflecting a compound annual growth rate (CAGR) of 4.4% over the forecast period. This growth is indicative of the increasing demand for polio vaccines as part of global health initiatives aimed at eradicating the disease. In comparison, the broader global pharmaceutical market was valued at $1,475 billion in 2022, with a projected CAGR of 5% over the next six years. This highlights the significant scale and growth potential of the pharmaceutical industry as a whole. Meanwhile, the chemical drug market, a subset of the pharmaceutical industry, was estimated to grow from $1,005 billion in 2018 to $1,094 billion by 2022. These figures underscore the dynamic nature of the pharmaceutical and vaccine markets, driven by ongoing research, development, and innovation. The growth of the Inactivated Polio Virus Vaccine Market is supported by efforts to increase vaccine accessibility and coverage, particularly in regions where polio remains a threat. The market's expansion is also influenced by technological advancements, policy initiatives, and international collaborations aimed at achieving a polio-free world.
| Report Metric | Details |
| Report Name | Inactivated Polio Virus Vaccine Market |
| Accounted market size in year | US$ 744 million |
| Forecasted market size in 2031 | US$ 1002 million |
| CAGR | 4.4% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Sanofi, GSK, Serum Institute (Bilthoven Biologicals), IMBCA |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
