What is Global Drug for Organ Rejection Prophylaxis Market?
The Global Drug for Organ Rejection Prophylaxis Market is a specialized segment within the pharmaceutical industry that focuses on medications designed to prevent the rejection of transplanted organs. Organ rejection occurs when the recipient's immune system identifies the transplanted organ as foreign and attacks it, potentially leading to organ failure. To counteract this, drugs for organ rejection prophylaxis are administered to suppress the immune response, thereby increasing the chances of transplant success. These drugs are crucial for patients who have undergone organ transplants, such as kidney, liver, heart, or lung transplants, as they help maintain the functionality of the transplanted organ and improve the patient's quality of life. The market for these drugs is driven by the increasing number of organ transplant procedures worldwide, advancements in medical technology, and the growing prevalence of chronic diseases that lead to organ failure. As a result, the demand for effective immunosuppressive therapies continues to rise, making this market a vital component of the healthcare industry.
Oral Liquid, Injection, Capsule in the Global Drug for Organ Rejection Prophylaxis Market:
In the Global Drug for Organ Rejection Prophylaxis Market, various forms of medication are available, including oral liquids, injections, and capsules, each with its unique advantages and applications. Oral liquid formulations are particularly beneficial for patients who have difficulty swallowing pills, such as children or elderly individuals. These liquids allow for easy administration and dosage adjustments, ensuring that patients receive the precise amount of medication needed to prevent organ rejection. Additionally, oral liquids can be flavored to improve palatability, making them more acceptable for long-term use. On the other hand, injections are often used in acute settings or when rapid immunosuppression is required. Injectable drugs provide a direct and immediate effect, which is crucial in preventing acute rejection episodes shortly after transplantation. They are typically administered in a hospital or clinical setting under the supervision of healthcare professionals to ensure proper dosage and monitoring. Capsules, meanwhile, offer a convenient and portable option for patients who require long-term immunosuppressive therapy. They are easy to store and transport, making them ideal for patients who need to maintain their medication regimen while traveling or leading an active lifestyle. Capsules also provide a controlled release of medication, ensuring a steady level of immunosuppression over time. Each of these formulations plays a critical role in the comprehensive management of organ transplant patients, allowing for tailored treatment plans that meet the specific needs of individual patients. The choice of formulation depends on various factors, including the patient's medical condition, lifestyle, and personal preferences, as well as the specific requirements of the transplanted organ. By offering a range of options, the Global Drug for Organ Rejection Prophylaxis Market ensures that patients have access to the most appropriate and effective therapies for their unique circumstances.
Hospital, Specialist Clinic, Other in the Global Drug for Organ Rejection Prophylaxis Market:
The usage of drugs for organ rejection prophylaxis is widespread across various healthcare settings, including hospitals, specialist clinics, and other medical facilities. In hospitals, these drugs are an integral part of the post-transplant care regimen. Hospitals are often the primary sites for organ transplantation procedures, and they have the necessary infrastructure and expertise to manage complex cases. After a transplant, patients typically remain in the hospital for a period of time to ensure that the transplanted organ is functioning properly and that there are no signs of rejection. During this time, healthcare professionals closely monitor the patient's response to immunosuppressive therapy and make any necessary adjustments to the medication regimen. Hospitals also provide education and support to patients and their families, helping them understand the importance of adherence to the prescribed treatment plan. Specialist clinics, on the other hand, offer ongoing care and monitoring for transplant patients after they are discharged from the hospital. These clinics are staffed by healthcare professionals with expertise in transplant medicine, who work closely with patients to manage their long-term immunosuppressive therapy. Regular follow-up visits to specialist clinics allow for the early detection and management of potential complications, such as infections or side effects from the medication. In addition to hospitals and specialist clinics, other healthcare facilities, such as outpatient centers and community health organizations, also play a role in the management of organ rejection prophylaxis. These facilities provide accessible and convenient care for patients who may not require the intensive monitoring available in hospitals or specialist clinics. They offer services such as medication counseling, routine blood tests, and support groups, which are essential for maintaining the health and well-being of transplant patients. By providing a continuum of care across different healthcare settings, the Global Drug for Organ Rejection Prophylaxis Market ensures that patients receive comprehensive and coordinated care throughout their transplant journey.
Global Drug for Organ Rejection Prophylaxis Market Outlook:
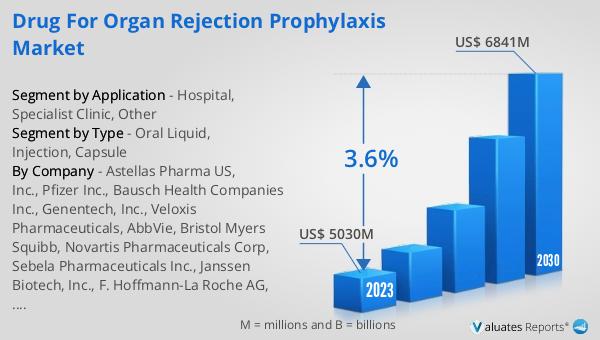
The global market for drugs used in organ rejection prophylaxis was valued at $5,712 million in 2024 and is anticipated to grow to a revised size of $7,291 million by 2031, reflecting a compound annual growth rate (CAGR) of 3.6% over the forecast period. This growth is indicative of the increasing demand for effective immunosuppressive therapies as the number of organ transplant procedures continues to rise worldwide. The market's expansion is driven by several factors, including advancements in medical technology, the growing prevalence of chronic diseases that lead to organ failure, and the development of new and improved drug formulations. As healthcare systems around the world strive to improve patient outcomes and quality of life for transplant recipients, the need for reliable and effective organ rejection prophylaxis drugs becomes ever more critical. The projected growth of this market underscores the importance of continued research and innovation in the field of transplant medicine, as well as the need for healthcare providers to remain informed about the latest developments in immunosuppressive therapy. By investing in the development and distribution of these essential medications, the Global Drug for Organ Rejection Prophylaxis Market plays a vital role in supporting the health and well-being of transplant patients worldwide.
| Report Metric | Details |
| Report Name | Drug for Organ Rejection Prophylaxis Market |
| Accounted market size in year | US$ 5712 million |
| Forecasted market size in 2031 | US$ 7291 million |
| CAGR | 3.6% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Astellas Pharma US, Inc., Pfizer Inc., Bausch Health Companies Inc., Genentech, Inc., Veloxis Pharmaceuticals, AbbVie, Bristol Myers Squibb, Novartis Pharmaceuticals Corp, Sebela Pharmaceuticals Inc., Janssen Biotech, Inc., F. Hoffmann-La Roche AG, Adienne Pharma & Biotech, Sanofi Genzyme Company, Heumann Pharma GmbH, Beijing Shuanglu Pharmaceutical Co., Ltd, North China Pharmaceutical Co., Ltd, Hangzhou Zhongmei Huadong Pharmaceutical Co., Ltd, Tianjin Central Pharmaceutical Co., Ltd, Nantong Jingjing Pharmaceutical Co., Ltd, Shanghai Pharmaceutical Group |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |