What is Global In Vitro Diagnostics (IVD) Quality Control Product Market?
The Global In Vitro Diagnostics (IVD) Quality Control Product Market is a vast and complex field that plays a crucial role in the healthcare industry. It refers to the global market for products and services that are used to ensure the quality and accuracy of In Vitro Diagnostics tests. These tests are performed on samples such as blood or tissue that have been taken from the human body. The IVD Quality Control Product Market includes a wide range of products such as control sera, reagents, and calibrators, which are used to check the precision and accuracy of IVD instruments and methods. The market also includes software solutions for data management and analysis. The global IVD Quality Control Product Market is driven by the increasing prevalence of chronic and infectious diseases, the growing geriatric population, and the rising demand for personalized medicine.
Quality Control Products, Quality Assurance Services, Data Management Solutions in the Global In Vitro Diagnostics (IVD) Quality Control Product Market:
Quality Control Products, Quality Assurance Services, and Data Management Solutions are integral components of the Global In Vitro Diagnostics (IVD) Quality Control Product Market. Quality Control Products include control sera, reagents, and calibrators that are used to ensure the accuracy and precision of IVD tests. These products are used in various stages of the testing process, from sample preparation to result interpretation. Quality Assurance Services, on the other hand, involve the systematic monitoring and evaluation of various aspects of a project, service, or facility to ensure that standards of quality are being met. These services may include auditing, training, and certification. Data Management Solutions refer to software and services that are used to manage, process, and analyze large volumes of data generated by IVD tests. These solutions help in improving the efficiency and accuracy of IVD tests, thereby enhancing patient care.
Clinical Chemistry, Immunochemistry, Hematology, Molecular Diagnostics, Others in the Global In Vitro Diagnostics (IVD) Quality Control Product Market:
The Global In Vitro Diagnostics (IVD) Quality Control Product Market finds extensive application in various areas such as Clinical Chemistry, Immunochemistry, Hematology, Molecular Diagnostics, and others. In Clinical Chemistry, IVD Quality Control Products are used to ensure the accuracy of tests that measure the levels of various chemical substances in the body. In Immunochemistry, these products are used to verify the precision of tests that detect and measure specific proteins or other substances through their properties as antigens or antibodies. In Hematology, IVD Quality Control Products are used to check the accuracy of tests that analyze blood cells and their components. In Molecular Diagnostics, these products are used to ensure the precision of tests that detect specific sequences in DNA or RNA that may or may not be associated with disease. In other areas, IVD Quality Control Products are used to maintain the quality and accuracy of various other types of diagnostic tests.
Global In Vitro Diagnostics (IVD) Quality Control Product Market Outlook:
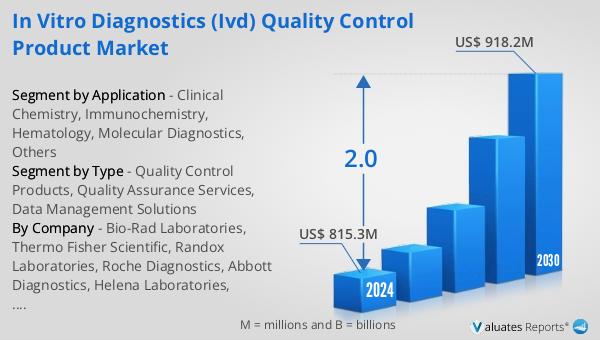
The outlook for the Global In Vitro Diagnostics (IVD) Quality Control Product Market is promising. In 2023, the market was valued at US$ 776 million and it is expected to reach US$ 918.2 million by 2030, growing at a Compound Annual Growth Rate (CAGR) of 2.0% during the forecast period from 2024 to 2030. This growth is driven by various factors such as the increasing prevalence of chronic and infectious diseases, the growing geriatric population, and the rising demand for personalized medicine. In comparison, the global pharmaceutical market, which was worth 1475 billion USD in 2022, is expected to grow at a CAGR of 5% over the next six years. The chemical drug market, on the other hand, is estimated to increase from 1005 billion in 2018 to 1094 billion U.S. dollars in 2022.
| Report Metric | Details |
| Report Name | In Vitro Diagnostics (IVD) Quality Control Product Market |
| Accounted market size in 2023 | US$ 776 million |
| Forecasted market size in 2030 | US$ 918.2 million |
| CAGR | 2.0% |
| Base Year | 2023 |
| Forecasted years | 2024 - 2030 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | Bio-Rad Laboratories, Thermo Fisher Scientific, Randox Laboratories, Roche Diagnostics, Abbott Diagnostics, Helena Laboratories, Seracare Life Sciences, Technopath Clinical Diagnostics, Sun Diagnostics, Zeptometrix Corporation, ISOLAB, Sysmex Corporation, Fortress Diagnostics, Meril Life Sciences, Multiplicom, Future Diagnostics Solutions, Surmodics |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |