What is Global eCOA and ePRO Solutions for Clinical Trials Market?
The Global eCOA (Electronic Clinical Outcome Assessment) and ePRO (Electronic Patient Reported Outcomes) Solutions for Clinical Trials Market is a rapidly evolving field that is transforming the way clinical trials are conducted. These solutions are digital platforms that capture patient data in real time, enabling researchers to monitor patient health and treatment outcomes more accurately and efficiently. The eCOA and ePRO solutions are designed to streamline the data collection process, reduce errors, and improve the quality of data collected in clinical trials. They are used in a variety of clinical trials, including those for new drugs, medical devices, and other therapeutic interventions. These solutions are particularly beneficial in large-scale, multi-site trials where data consistency and accuracy are paramount. However, the adoption of these solutions is not without challenges. The high cost of implementation, lack of technical expertise, and data security concerns are some of the factors that may hinder market growth. Despite these challenges, the benefits offered by eCOA and ePRO solutions, such as improved data quality, real-time access to patient data, and increased patient engagement, are expected to drive their adoption in the coming years.
On Premise, Cloud-based in the Global eCOA and ePRO Solutions for Clinical Trials Market:
On Premise and Cloud-based eCOA and ePRO solutions are two different types of deployment models used in the Global eCOA and ePRO Solutions for Clinical Trials Market. On Premise solutions are installed and run on computers on the premises of the organization using the software, rather than at a remote facility such as a server farm or cloud. On the other hand, Cloud-based solutions are hosted on the vendor's servers and accessed through the internet. Both these models have their own advantages and disadvantages. On Premise solutions offer more control over the data and are considered more secure as the data remains within the organization's premises. However, they require a significant upfront investment in hardware and software, and ongoing costs for maintenance and upgrades. Cloud-based solutions, on the other hand, are more cost-effective as they eliminate the need for upfront hardware and software investment. They offer scalability and flexibility, allowing organizations to easily scale up or down based on their needs. However, data security can be a concern with cloud-based solutions as the data is stored on the vendor's servers. Despite these challenges, the adoption of both On Premise and Cloud-based eCOA and ePRO solutions is expected to grow in the coming years, driven by the increasing need for efficient and accurate data collection in clinical trials.
Hospitals, Pharmaceutical & Biotechnology, Others in the Global eCOA and ePRO Solutions for Clinical Trials Market:
The Global eCOA and ePRO Solutions for Clinical Trials Market finds extensive usage in various areas such as Hospitals, Pharmaceutical & Biotechnology companies, and others. In hospitals, these solutions are used to collect patient data in real time, enabling healthcare professionals to monitor patient health and treatment outcomes more accurately and efficiently. They help in reducing errors and improving the quality of data collected, thereby enhancing patient care. In the Pharmaceutical & Biotechnology industry, eCOA and ePRO solutions are used in clinical trials for new drugs and therapeutic interventions. They streamline the data collection process, ensuring data consistency and accuracy, which is crucial in large-scale, multi-site trials. Other areas where these solutions find usage include research institutions and contract research organizations. Despite the challenges such as high implementation costs and data security concerns, the benefits offered by eCOA and ePRO solutions, such as improved data quality, real-time access to patient data, and increased patient engagement, are expected to drive their adoption in these areas.
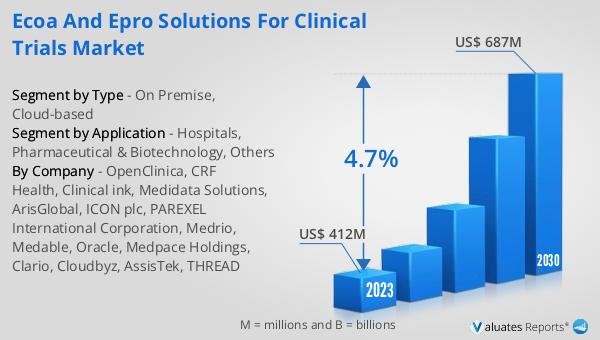
Global eCOA and ePRO Solutions for Clinical Trials Market Outlook:
The Global eCOA and ePRO Solutions for Clinical Trials Market has shown significant growth in recent years. In 2022, the market was valued at US$ 523 million. It is projected to reach a value of US$ 687 million by 2029. This represents a Compound Annual Growth Rate (CAGR) of 4.7% during the forecast period from 2023 to 2029. This growth can be attributed to several factors. The increasing adoption of digital platforms in healthcare, the growing need for real-time patient data in clinical trials, and the benefits offered by eCOA and ePRO solutions, such as improved data quality and increased patient engagement, are some of the key factors driving the market growth. However, challenges such as high implementation costs, lack of technical expertise, and data security concerns may hinder market growth. Despite these challenges, the market is expected to witness significant growth in the coming years, driven by the increasing need for efficient and accurate data collection in clinical trials.
| Report Metric | Details |
| Report Name | eCOA and ePRO Solutions for Clinical Trials Market |
| Accounted market size in 2022 | US$ 523 in million |
| Forecasted market size in 2029 | US$ 687 million |
| CAGR | 4.7% |
| Base Year | 2022 |
| Forecasted years | 2023 - 2029 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | OpenClinica, CRF Health, Clinical ink, Medidata Solutions, ArisGlobal, ICON plc, PAREXEL International Corporation, Medrio, Medable, Oracle, Medpace Holdings, Clario, Cloudbyz, AssisTek, THREAD |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
