What is Global Oxcarbazepine Drug Market?
The global Oxcarbazepine drug market is a significant segment within the pharmaceutical industry, primarily focused on the production and distribution of Oxcarbazepine, a medication used to treat epilepsy and certain types of seizures. Oxcarbazepine works by stabilizing electrical activity in the brain, thereby reducing the frequency and intensity of seizures. This drug is particularly important for patients who do not respond well to other antiepileptic medications. The market for Oxcarbazepine is driven by the increasing prevalence of epilepsy worldwide, advancements in drug formulations, and a growing awareness of neurological disorders. Additionally, the market benefits from ongoing research and development efforts aimed at improving the efficacy and safety of Oxcarbazepine. As healthcare systems globally continue to prioritize the treatment of neurological conditions, the demand for effective antiepileptic drugs like Oxcarbazepine is expected to rise. This market is characterized by a mix of generic and branded products, with key players investing in marketing strategies to expand their reach. The global Oxcarbazepine drug market is poised for growth, reflecting broader trends in the pharmaceutical industry and the increasing need for specialized medications.
Tablet, Oral Suspension in the Global Oxcarbazepine Drug Market:
In the global Oxcarbazepine drug market, two primary formulations are widely used: tablets and oral suspensions. Tablets are the most common form, offering convenience and ease of administration. They are typically prescribed for adults and older children who can swallow pills. The tablet form of Oxcarbazepine is designed to release the medication gradually, ensuring a steady concentration in the bloodstream, which helps in maintaining consistent therapeutic effects. This formulation is particularly beneficial for patients who require long-term management of epilepsy, as it allows for precise dosing and adherence to treatment regimens. On the other hand, oral suspensions provide an alternative for patients who have difficulty swallowing tablets, such as young children or the elderly. The suspension form is a liquid that can be easily measured and administered, making it suitable for pediatric use. It allows for flexible dosing, which is crucial for tailoring treatment to individual needs, especially in children whose dosage requirements may change as they grow. The availability of both tablets and oral suspensions in the Oxcarbazepine market ensures that a wide range of patients can access this essential medication, regardless of age or swallowing ability. The choice between these formulations often depends on patient preference, age, and specific medical needs. Healthcare providers play a crucial role in determining the most appropriate form of Oxcarbazepine for each patient, taking into account factors such as the severity of the condition, potential side effects, and the patient's overall health status. In recent years, there has been a growing emphasis on patient-centered care, which has led to increased demand for diverse drug formulations that cater to individual preferences and requirements. This trend is evident in the Oxcarbazepine market, where manufacturers are continually exploring new ways to enhance the delivery and efficacy of their products. For instance, advancements in drug delivery technologies have led to the development of extended-release tablets, which offer the convenience of once-daily dosing. This innovation not only improves patient compliance but also enhances the overall effectiveness of the treatment. Similarly, improvements in the formulation of oral suspensions have resulted in better taste and stability, making them more palatable for children. The global Oxcarbazepine drug market is also influenced by regulatory considerations, as pharmaceutical companies must adhere to stringent guidelines to ensure the safety and efficacy of their products. This includes conducting rigorous clinical trials and obtaining necessary approvals from regulatory bodies before bringing new formulations to market. As a result, the development and commercialization of Oxcarbazepine tablets and oral suspensions involve significant investment in research and development, quality control, and marketing efforts. Despite these challenges, the market for Oxcarbazepine continues to expand, driven by the increasing prevalence of epilepsy and the growing demand for effective antiepileptic medications. The availability of multiple formulations, such as tablets and oral suspensions, ensures that patients have access to tailored treatment options that meet their specific needs. This diversity in drug formulations is a testament to the pharmaceutical industry's commitment to improving patient outcomes and enhancing the quality of life for individuals living with epilepsy. As the global Oxcarbazepine drug market evolves, it is likely to see further innovations in drug delivery and formulation, paving the way for more effective and patient-friendly treatment options.
Adult, Pediatric in the Global Oxcarbazepine Drug Market:
The usage of Oxcarbazepine in the global market varies significantly between adult and pediatric populations, reflecting the diverse needs and treatment considerations for these groups. In adults, Oxcarbazepine is primarily used to manage partial seizures, which are common in epilepsy. The drug's mechanism of action involves stabilizing neuronal membranes and inhibiting repetitive firing, which helps reduce seizure frequency and severity. For adult patients, Oxcarbazepine is often prescribed as a monotherapy or as an adjunctive therapy in combination with other antiepileptic drugs. The choice of therapy depends on the individual's medical history, seizure type, and response to previous treatments. Adults typically receive Oxcarbazepine in tablet form, which allows for precise dosing and ease of administration. The drug's effectiveness in controlling seizures has made it a preferred choice for many healthcare providers, particularly for patients who have not responded well to other medications. In the pediatric population, Oxcarbazepine is used to treat partial seizures in children as young as two years old. The drug's safety and efficacy in children have been well-documented, making it a valuable option for managing epilepsy in this age group. Pediatric patients often require different dosing considerations compared to adults, as their bodies metabolize drugs differently. As a result, healthcare providers must carefully adjust Oxcarbazepine dosages based on the child's weight, age, and overall health status. Oral suspensions are commonly used for pediatric patients, as they allow for flexible dosing and are easier for children to ingest. The liquid form of Oxcarbazepine can be precisely measured, ensuring that young patients receive the appropriate dose for their needs. This flexibility is crucial for managing epilepsy in children, as their dosage requirements may change over time due to growth and development. The use of Oxcarbazepine in both adult and pediatric populations highlights the importance of personalized medicine in the treatment of epilepsy. Healthcare providers must consider a range of factors, including age, seizure type, and individual response to medication, when developing treatment plans for patients. This personalized approach ensures that each patient receives the most effective and appropriate therapy for their condition. The global Oxcarbazepine drug market continues to evolve, with ongoing research and development efforts aimed at improving the drug's safety and efficacy for both adults and children. As new formulations and delivery methods are developed, patients can expect to see even more tailored treatment options that address their unique needs. The availability of Oxcarbazepine in various forms, such as tablets and oral suspensions, ensures that patients of all ages have access to effective epilepsy management. This diversity in drug formulations is a testament to the pharmaceutical industry's commitment to improving patient outcomes and enhancing the quality of life for individuals living with epilepsy. As the global Oxcarbazepine drug market continues to grow, it is likely to see further innovations in drug delivery and formulation, paving the way for more effective and patient-friendly treatment options for both adult and pediatric populations.
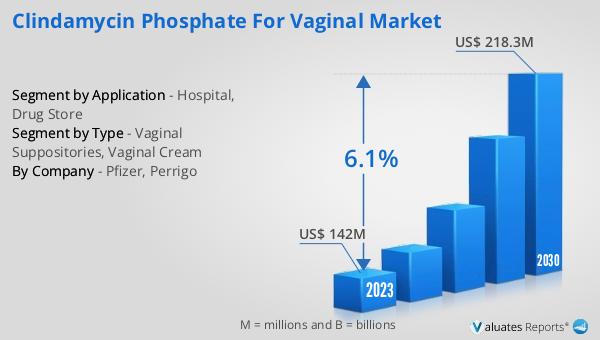
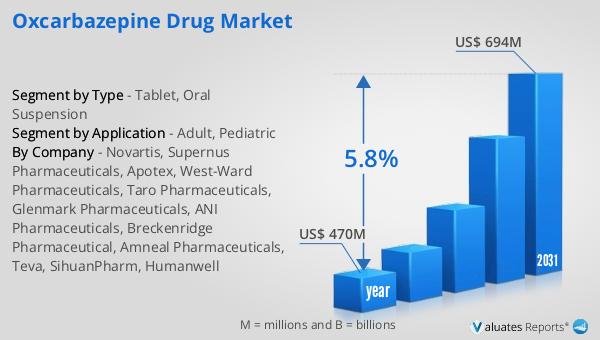
Global Oxcarbazepine Drug Market Outlook:
The global Oxcarbazepine drug market was valued at approximately $470 million in 2024 and is anticipated to expand to a revised size of $694 million by 2031, reflecting a compound annual growth rate (CAGR) of 5.8% over the forecast period. This growth trajectory underscores the increasing demand for Oxcarbazepine as an effective treatment for epilepsy and related neurological disorders. In the broader context, the global pharmaceutical market was valued at $1,475 billion in 2022, with an expected CAGR of 5% over the next six years. This indicates a robust growth pattern within the pharmaceutical sector, driven by advancements in drug development, increasing healthcare expenditure, and a growing focus on personalized medicine. Comparatively, the chemical drug market has shown a steady increase, rising from $1,005 billion in 2018 to $1,094 billion in 2022. This growth is indicative of the sustained demand for chemical-based medications, including Oxcarbazepine, which remains a critical component of epilepsy treatment regimens. The expansion of the Oxcarbazepine market is reflective of broader trends within the pharmaceutical industry, where there is a continuous push towards developing innovative therapies that address unmet medical needs. As healthcare systems worldwide continue to prioritize the treatment of neurological conditions, the demand for effective antiepileptic drugs like Oxcarbazepine is expected to rise, contributing to the market's overall growth.
| Report Metric | Details |
| Report Name | Oxcarbazepine Drug Market |
| Accounted market size in year | US$ 470 million |
| Forecasted market size in 2031 | US$ 694 million |
| CAGR | 5.8% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Novartis, Supernus Pharmaceuticals, Apotex, West-Ward Pharmaceuticals, Taro Pharmaceuticals, Glenmark Pharmaceuticals, ANI Pharmaceuticals, Breckenridge Pharmaceutical, Amneal Pharmaceuticals, Teva, SihuanPharm, Humanwell |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |