What is Global In-Mould Labels (IML) Market?
The Global In-Mould Labels (IML) Market refers to the industry focused on the production and application of labels that are integrated into plastic containers during the manufacturing process. This innovative labeling technique involves placing a pre-printed label inside a mold before injecting the plastic, allowing the label to become an integral part of the final product. This method offers several advantages, such as enhanced durability, improved aesthetics, and resistance to moisture and scratches. IML is widely used in various sectors, including food and beverages, chemicals, and healthcare, due to its ability to provide high-quality, visually appealing packaging solutions. The market for IML is driven by the growing demand for sustainable and efficient packaging options, as well as the increasing need for product differentiation in competitive markets. As industries continue to seek innovative ways to enhance their brand image and consumer appeal, the Global In-Mould Labels Market is expected to experience significant growth, offering numerous opportunities for manufacturers and suppliers in the coming years.
Injection Molding, Extrusion- Blow Molding, Thermoforming in the Global In-Mould Labels (IML) Market:
Injection molding, extrusion-blow molding, and thermoforming are three key processes utilized in the Global In-Mould Labels (IML) Market, each offering unique advantages for integrating labels into plastic products. Injection molding is a widely used manufacturing process where molten plastic is injected into a mold cavity, which contains the in-mould label. As the plastic cools and solidifies, the label becomes an integral part of the product. This method is particularly beneficial for producing complex shapes and detailed designs, making it ideal for creating high-quality, durable packaging solutions. The precision and efficiency of injection molding make it a popular choice for industries requiring large volumes of consistent, high-quality products. Extrusion-blow molding, on the other hand, involves extruding a tube of molten plastic, known as a parison, into a mold. Air is then blown into the parison, expanding it to conform to the shape of the mold, with the in-mould label becoming embedded in the process. This technique is particularly suited for producing hollow objects, such as bottles and containers, and is favored for its ability to create lightweight, yet sturdy products. The integration of IML in extrusion-blow molding enhances the aesthetic appeal and functionality of the final product, making it a preferred choice for packaging in the food and beverage industry. Thermoforming is another process used in the IML market, involving the heating of a plastic sheet until it becomes pliable, then forming it over a mold with the in-mould label in place. The plastic cools and hardens, with the label becoming a permanent part of the product. This method is advantageous for producing large, thin-walled items and is often used for packaging applications where cost-effectiveness and speed are crucial. The versatility of thermoforming allows for the production of a wide range of products, from simple trays to complex packaging solutions, with the added benefit of high-quality labeling. Each of these processes plays a vital role in the Global In-Mould Labels Market, offering manufacturers the flexibility to choose the most suitable method for their specific needs, while ensuring the production of visually appealing and durable products. As the demand for innovative and sustainable packaging solutions continues to grow, these processes will remain integral to the advancement of the IML market.
Food & Beverages, Chemicals, Healthcare, Others in the Global In-Mould Labels (IML) Market:
The Global In-Mould Labels (IML) Market finds extensive usage across various sectors, including food and beverages, chemicals, healthcare, and others, due to its ability to provide high-quality, durable, and visually appealing packaging solutions. In the food and beverages industry, IML is particularly valued for its ability to enhance product presentation and shelf appeal. The integration of labels during the manufacturing process ensures that they are resistant to moisture, scratches, and other environmental factors, making them ideal for packaging perishable goods. This durability, combined with the ability to create intricate designs and vibrant colors, allows brands to effectively communicate their identity and attract consumers. In the chemicals sector, IML offers significant advantages in terms of safety and compliance. The labels can include important information such as hazard warnings, usage instructions, and regulatory compliance details, which are crucial for ensuring the safe handling and use of chemical products. The durability of IML ensures that this information remains intact and legible throughout the product's lifecycle, even in harsh environments. In the healthcare industry, IML is used to create packaging that meets stringent hygiene and safety standards. The labels can include essential information such as dosage instructions, expiration dates, and batch numbers, which are critical for patient safety and regulatory compliance. The integration of labels during the manufacturing process ensures that they are tamper-proof and resistant to contamination, making them ideal for packaging pharmaceuticals and medical devices. Beyond these sectors, IML is also used in a variety of other applications, including personal care products, household goods, and automotive parts. The versatility and durability of IML make it a preferred choice for manufacturers seeking to enhance their brand image and product appeal. As industries continue to prioritize sustainability and innovation in packaging, the Global In-Mould Labels Market is expected to see continued growth and adoption across a wide range of applications.
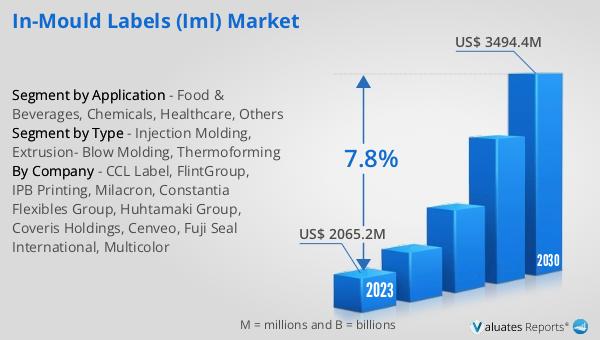
Global In-Mould Labels (IML) Market Outlook:
The global market for In-Mould Labels (IML) was valued at approximately USD 2,383 million in 2024, and it is anticipated to expand to a revised size of around USD 4,002 million by 2031. This growth trajectory represents a compound annual growth rate (CAGR) of 7.8% over the forecast period. The increasing demand for innovative and sustainable packaging solutions is a key driver of this market expansion. As industries across the globe seek to enhance their product differentiation and brand appeal, the adoption of IML is expected to rise significantly. The integration of labels during the manufacturing process offers numerous benefits, including enhanced durability, improved aesthetics, and resistance to environmental factors, making it an attractive option for various sectors. The food and beverages industry, in particular, is expected to be a major contributor to the growth of the IML market, as brands seek to create visually appealing and durable packaging solutions that can withstand the rigors of transportation and storage. Additionally, the chemicals and healthcare sectors are anticipated to drive demand for IML, as they require packaging solutions that meet stringent safety and compliance standards. As the market continues to evolve, manufacturers and suppliers in the IML industry are likely to explore new opportunities and innovations to meet the growing demand for high-quality, sustainable packaging solutions.
| Report Metric | Details |
| Report Name | In-Mould Labels (IML) Market |
| Accounted market size in year | US$ 2383 million |
| Forecasted market size in 2031 | US$ 4002 million |
| CAGR | 7.8% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | CCL Label, FlintGroup, IPB Printing, Milacron, Constantia Flexibles Group, Huhtamaki Group, Coveris Holdings, Cenveo, Fuji Seal International, Multicolor |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |