What is Global Pharmaceutical Grade Eribulin Mesylate Market?
The Global Pharmaceutical Grade Eribulin Mesylate Market refers to the worldwide industry focused on the production and distribution of Eribulin Mesylate, a chemotherapy drug used primarily in the treatment of metastatic breast cancer and liposarcoma. This market is driven by the increasing prevalence of cancer, advancements in pharmaceutical research, and the growing demand for effective cancer treatments. Eribulin Mesylate works by inhibiting the growth of cancer cells, thereby slowing down or stopping the progression of the disease. The market encompasses various stakeholders, including pharmaceutical companies, research institutions, healthcare providers, and regulatory bodies, all working together to ensure the availability and accessibility of this critical medication. As cancer remains a leading cause of mortality globally, the demand for effective treatments like Eribulin Mesylate continues to rise, making this market an essential component of the broader pharmaceutical industry. The market's growth is also influenced by factors such as government initiatives, healthcare infrastructure development, and increasing awareness about cancer treatment options. Overall, the Global Pharmaceutical Grade Eribulin Mesylate Market plays a crucial role in addressing the global cancer burden and improving patient outcomes.
Purity≥99%, Purity<99% in the Global Pharmaceutical Grade Eribulin Mesylate Market:
In the Global Pharmaceutical Grade Eribulin Mesylate Market, the purity of the drug is a critical factor that determines its efficacy and safety. Purity levels are typically categorized into two main segments: Purity≥99% and Purity<99%. The Purity≥99% segment represents Eribulin Mesylate that has been refined to a high degree of purity, ensuring that it contains minimal impurities and contaminants. This high-purity form is often preferred in clinical settings due to its enhanced effectiveness and reduced risk of adverse reactions. Pharmaceutical companies invest significant resources in refining their production processes to achieve this level of purity, as it directly impacts the drug's therapeutic potential. On the other hand, the Purity<99% segment includes Eribulin Mesylate with slightly lower purity levels. While still effective, this form may contain a higher concentration of impurities, which can affect its overall performance and safety profile. The choice between these two purity levels often depends on factors such as cost, intended use, and regulatory requirements. In many cases, the Purity≥99% form is used in critical applications where the highest level of efficacy is required, while the Purity<99% form may be utilized in less demanding scenarios. The distinction between these two purity levels highlights the importance of quality control and rigorous testing in the pharmaceutical industry. Companies must adhere to strict guidelines and standards to ensure that their products meet the necessary purity criteria, thereby safeguarding patient health and maintaining regulatory compliance. As the demand for effective cancer treatments continues to grow, the focus on purity in the Eribulin Mesylate market is likely to intensify, driving further advancements in production techniques and quality assurance measures. This emphasis on purity not only enhances the drug's therapeutic potential but also reinforces the industry's commitment to delivering safe and reliable medications to patients worldwide.
Solution, Others in the Global Pharmaceutical Grade Eribulin Mesylate Market:
The usage of Global Pharmaceutical Grade Eribulin Mesylate Market extends to various applications, including solutions and other forms. In the context of solutions, Eribulin Mesylate is often formulated into injectable solutions for direct administration to patients. This form of delivery is particularly effective in ensuring that the drug reaches the bloodstream quickly, allowing for rapid therapeutic action. Injectable solutions are commonly used in clinical settings, where healthcare professionals can closely monitor the patient's response to treatment and adjust dosages as needed. The precision and control offered by injectable solutions make them a preferred choice for administering Eribulin Mesylate, especially in cases where immediate intervention is required. Beyond solutions, Eribulin Mesylate is also utilized in other forms, such as oral formulations or combination therapies. These alternative forms offer flexibility in treatment regimens, catering to the diverse needs of patients and healthcare providers. Oral formulations, for example, provide a convenient option for patients who may have difficulty with injections or require long-term maintenance therapy. Combination therapies, on the other hand, involve using Eribulin Mesylate alongside other cancer treatments to enhance overall efficacy and improve patient outcomes. This approach is particularly beneficial in complex cases where a multi-faceted treatment strategy is necessary to combat the disease effectively. The versatility of Eribulin Mesylate in various forms underscores its significance in the global pharmaceutical landscape. As research continues to explore new applications and delivery methods, the potential for Eribulin Mesylate to address a broader range of cancer types and patient needs is likely to expand. This ongoing innovation not only enhances the drug's therapeutic value but also reinforces its role as a vital component of modern cancer treatment protocols.
Global Pharmaceutical Grade Eribulin Mesylate Market Outlook:
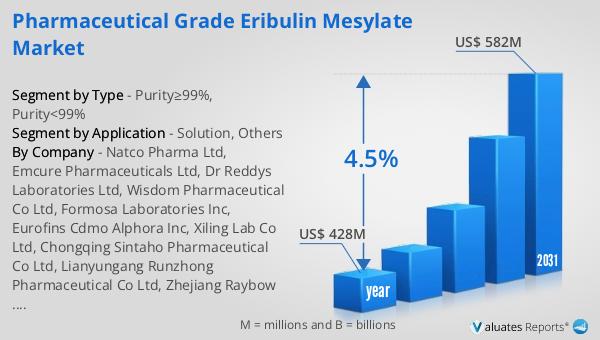
The global market for Pharmaceutical Grade Eribulin Mesylate was valued at $428 million in 2024 and is anticipated to grow to a revised size of $582 million by 2031, reflecting a compound annual growth rate (CAGR) of 4.5% during the forecast period. This growth trajectory underscores the increasing demand for effective cancer treatments and the pivotal role of Eribulin Mesylate in addressing this need. In comparison, the broader global pharmaceutical market was valued at $1,475 billion in 2022, with a projected CAGR of 5% over the next six years. This indicates a robust expansion across the pharmaceutical sector, driven by factors such as technological advancements, increased healthcare spending, and a growing focus on personalized medicine. Meanwhile, the chemical drug market, a subset of the pharmaceutical industry, is estimated to have grown from $1,005 billion in 2018 to $1,094 billion in 2022. This growth highlights the sustained demand for chemical-based therapies, including Eribulin Mesylate, as essential components of modern healthcare. The comparative analysis of these markets illustrates the dynamic nature of the pharmaceutical landscape and the critical role of innovative treatments like Eribulin Mesylate in driving industry growth. As the market continues to evolve, stakeholders across the pharmaceutical value chain are likely to focus on enhancing drug efficacy, improving patient access, and fostering collaboration to address the global cancer burden effectively.
| Report Metric | Details |
| Report Name | Pharmaceutical Grade Eribulin Mesylate Market |
| Accounted market size in year | US$ 428 million |
| Forecasted market size in 2031 | US$ 582 million |
| CAGR | 4.5% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | Natco Pharma Ltd, Emcure Pharmaceuticals Ltd, Dr Reddys Laboratories Ltd, Wisdom Pharmaceutical Co Ltd, Formosa Laboratories Inc, Eurofins Cdmo Alphora Inc, Xiling Lab Co Ltd, Chongqing Sintaho Pharmaceutical Co Ltd, Lianyungang Runzhong Pharmaceutical Co Ltd, Zhejiang Raybow Pharmaceutical Co Ltd, Brightgene Bio-Medical Technology Co Ltd |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |