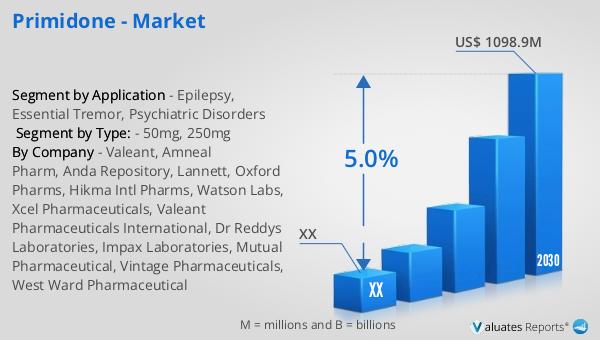
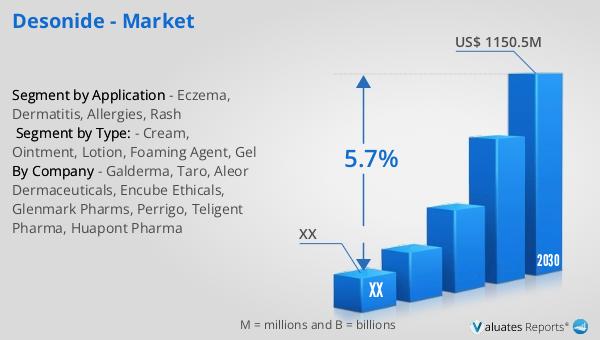
What is Desonide - Global Market?
Desonide is a low-potency topical corticosteroid used primarily to treat a variety of skin conditions. It is particularly effective in reducing inflammation, redness, and itching associated with conditions like eczema, dermatitis, allergies, and rashes. The global market for Desonide has been growing steadily due to its effectiveness and relatively low risk of side effects compared to more potent corticosteroids. In 2023, the market was valued at approximately US$ 782 million, and it is projected to reach around US$ 1150.5 million by 2030, reflecting a compound annual growth rate (CAGR) of 5.7% from 2024 to 2030. This growth is driven by increasing awareness of skin health, rising incidences of dermatological conditions, and the expanding pharmaceutical industry. As a part of the broader pharmaceutical market, which was valued at US$ 1475 billion in 2022, Desonide represents a small but significant segment, particularly within the topical corticosteroid category. The demand for Desonide is expected to continue rising as more people seek effective treatments for skin conditions, and as healthcare access improves globally.
Cream, Ointment, Lotion, Foaming Agent, Gel in the Desonide - Global Market:
Desonide is available in various formulations, including creams, ointments, lotions, foaming agents, and gels, each catering to different preferences and skin conditions. The cream formulation is perhaps the most commonly used due to its versatility and ease of application. It is suitable for most skin types and can be applied to both moist and dry areas, making it ideal for treating widespread skin conditions like eczema and dermatitis. The cream's emollient properties help to soothe and hydrate the skin while delivering the active ingredient effectively. Ointments, on the other hand, are thicker and more occlusive, making them suitable for very dry or scaly skin conditions. They provide a protective barrier that locks in moisture, which can be particularly beneficial for chronic conditions that require long-term treatment. Lotions are lighter and more fluid than creams, making them easier to spread over large areas of the body. They are often preferred for hairy areas or for treating conditions that cover extensive skin surfaces. Lotions are absorbed quickly and are less greasy, which can enhance patient compliance, especially in hot or humid climates. Foaming agents are a relatively newer formulation that offers a unique delivery system. They are easy to apply and spread evenly, providing a cooling sensation that can be soothing for inflamed or irritated skin. Foams are particularly useful for treating scalp conditions, as they can penetrate hair without leaving a residue. Gels are another option, known for their fast absorption and non-greasy feel. They are ideal for oily or acne-prone skin, as they do not add additional oil to the skin's surface. Gels can be particularly effective for treating localized areas of inflammation or irritation. Each of these formulations has its own advantages and is chosen based on the specific needs of the patient and the characteristics of the skin condition being treated. The availability of multiple formulations allows healthcare providers to tailor treatment to individual patient needs, enhancing the effectiveness of Desonide in managing various dermatological conditions. The global market for these formulations is expanding as more people seek personalized and effective treatments for their skin conditions. As awareness of the benefits of topical corticosteroids like Desonide grows, so too does the demand for these diverse formulations, contributing to the overall growth of the Desonide market.
Eczema, Dermatitis, Allergies, Rash in the Desonide - Global Market:
Desonide is widely used in the treatment of eczema, dermatitis, allergies, and rashes, owing to its anti-inflammatory and immunosuppressive properties. Eczema, a chronic skin condition characterized by itchy, inflamed patches of skin, can be effectively managed with Desonide. The medication helps to reduce the inflammation and itching, providing relief to patients and improving their quality of life. For those with dermatitis, which encompasses a range of skin irritations and inflammations, Desonide offers a reliable treatment option. It helps to calm the skin, reduce redness, and alleviate discomfort, making it a popular choice among dermatologists. Allergic reactions that manifest as skin rashes can also be treated with Desonide. By suppressing the immune response that causes the allergic reaction, Desonide helps to clear up the rash and prevent further irritation. This makes it an essential medication for individuals who experience frequent allergic skin reactions. Rashes, whether caused by allergies, infections, or other irritants, can be effectively treated with Desonide. Its ability to reduce inflammation and soothe the skin makes it a versatile treatment option for a variety of rash types. The global market for Desonide in these areas is driven by the increasing prevalence of skin conditions and the growing awareness of the importance of skin health. As more people seek effective treatments for their skin issues, the demand for Desonide continues to rise. Healthcare providers appreciate the flexibility that Desonide offers, with its range of formulations allowing for tailored treatment plans that meet the specific needs of each patient. This adaptability, combined with its proven efficacy, ensures that Desonide remains a key player in the global market for dermatological treatments.
Desonide - Global Market Outlook:
The global market outlook for Desonide indicates a promising future, with the market valued at approximately US$ 782 million in 2023. It is anticipated to grow to a revised size of US$ 1150.5 million by 2030, reflecting a compound annual growth rate (CAGR) of 5.7% during the forecast period from 2024 to 2030. This growth is indicative of the increasing demand for effective dermatological treatments and the expanding pharmaceutical industry. In comparison, the global pharmaceutical market was valued at US$ 1475 billion in 2022 and is expected to grow at a CAGR of 5% over the next six years. The chemical drug market, a significant segment of the pharmaceutical industry, was estimated to increase from US$ 1005 billion in 2018 to US$ 1094 billion in 2022. These figures highlight the robust growth of the pharmaceutical sector as a whole, with Desonide representing a small but important part of this market. The increasing prevalence of skin conditions, coupled with rising awareness of the importance of skin health, is driving the demand for Desonide. As healthcare access improves globally and more people seek effective treatments for their skin issues, the market for Desonide is expected to continue its upward trajectory. This growth is further supported by the availability of multiple formulations, which allow for personalized treatment plans that cater to the specific needs of each patient. Overall, the global market outlook for Desonide is positive, with steady growth anticipated in the coming years.
| Report Metric | Details |
| Report Name | Desonide - Market |
| Forecasted market size in 2030 | US$ 1150.5 million |
| CAGR | 5.7% |
| Forecasted years | 2024 - 2030 |
| Segment by Type: |
|
| Segment by Application |
|
| By Region |
|
| By Company | Galderma, Taro, Aleor Dermaceuticals, Encube Ethicals, Glenmark Pharms, Perrigo, Teligent Pharma, Huapont Pharma |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |