What is Global Budesonide Aerosol Market?
The Global Budesonide Aerosol Market refers to the worldwide industry focused on the production, distribution, and sale of budesonide aerosol products. Budesonide is a corticosteroid used primarily in the treatment of respiratory conditions such as asthma and chronic obstructive pulmonary disease (COPD). It works by reducing inflammation in the airways, making it easier for patients to breathe. The market for budesonide aerosol is driven by the increasing prevalence of respiratory diseases, advancements in inhalation technology, and a growing awareness of the benefits of early and effective treatment. As healthcare systems around the world continue to prioritize respiratory health, the demand for budesonide aerosol products is expected to rise. This market encompasses various stakeholders, including pharmaceutical companies, healthcare providers, and patients, all of whom play a role in the development and utilization of these treatments. The market is characterized by ongoing research and development efforts aimed at improving the efficacy and safety of budesonide formulations, as well as expanding their applications to a broader range of respiratory conditions. Overall, the Global Budesonide Aerosol Market is a dynamic and evolving sector that plays a crucial role in addressing the global burden of respiratory diseases.
50ug/200 Spray, 100ug/200 Spray, 200ug/100 Spray, Other in the Global Budesonide Aerosol Market:
In the Global Budesonide Aerosol Market, various formulations are available to cater to different patient needs and preferences. Among these, the 50ug/200 Spray, 100ug/200 Spray, and 200ug/100 Spray are prominent options, each offering distinct dosages and delivery mechanisms. The 50ug/200 Spray is typically used for patients who require a lower dose of budesonide, often in the early stages of treatment or for maintenance therapy. This formulation is designed to provide a consistent and controlled release of medication, ensuring that patients receive the appropriate amount of budesonide with each inhalation. The 100ug/200 Spray, on the other hand, offers a higher dosage per spray, making it suitable for patients with more severe symptoms or those who have not responded adequately to lower doses. This formulation is often prescribed for individuals who require a more intensive treatment regimen to manage their respiratory conditions effectively. The 200ug/100 Spray represents one of the highest dosages available in aerosol form, catering to patients with severe asthma or COPD who need a robust anti-inflammatory effect. This formulation is typically reserved for cases where other treatments have proven insufficient, providing a potent option for managing chronic respiratory conditions. In addition to these specific dosages, the market also includes other formulations that may combine budesonide with other medications or offer alternative delivery methods, such as dry powder inhalers or nebulizers. These options provide flexibility for healthcare providers to tailor treatment plans to individual patient needs, taking into account factors such as age, severity of symptoms, and comorbidities. The availability of multiple formulations within the Global Budesonide Aerosol Market underscores the importance of personalized medicine in respiratory care, allowing for more precise and effective management of conditions like asthma and COPD. As research continues to advance in this field, new formulations and delivery systems are likely to emerge, further enhancing the therapeutic options available to patients and healthcare providers.
Hospitals, Clinics, Other in the Global Budesonide Aerosol Market:
The usage of Global Budesonide Aerosol Market products spans various healthcare settings, including hospitals, clinics, and other medical facilities. In hospitals, budesonide aerosol is often used as part of a comprehensive treatment plan for patients with acute exacerbations of asthma or COPD. Hospitalized patients may receive budesonide aerosol in combination with other medications, such as bronchodilators or systemic corticosteroids, to achieve rapid symptom relief and stabilize their condition. The controlled environment of a hospital allows for close monitoring of the patient's response to treatment, enabling healthcare providers to make timely adjustments to the therapeutic regimen as needed. In clinics, budesonide aerosol is commonly prescribed for outpatient management of chronic respiratory conditions. Patients visiting clinics for routine check-ups or follow-up appointments may receive budesonide aerosol as part of their long-term maintenance therapy. The convenience of aerosol delivery makes it an attractive option for patients who require ongoing treatment but prefer to manage their condition outside of a hospital setting. Clinics play a crucial role in educating patients about proper inhaler technique and adherence to prescribed treatment plans, ensuring optimal outcomes. Beyond hospitals and clinics, budesonide aerosol is also utilized in other settings, such as home healthcare and telemedicine. Home healthcare providers may administer budesonide aerosol to patients who are unable to visit medical facilities due to mobility issues or other constraints. Telemedicine platforms have also emerged as a valuable tool for managing respiratory conditions remotely, allowing healthcare providers to prescribe and monitor budesonide aerosol treatment through virtual consultations. This approach offers increased accessibility and convenience for patients, particularly those living in rural or underserved areas. Overall, the versatility of budesonide aerosol in various healthcare settings highlights its importance as a cornerstone of respiratory care, providing effective treatment options for patients across the continuum of care.
Global Budesonide Aerosol Market Outlook:
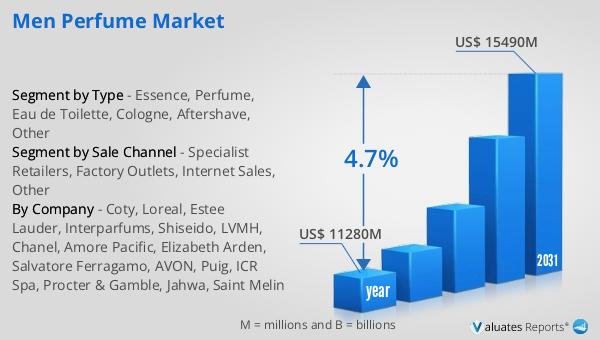
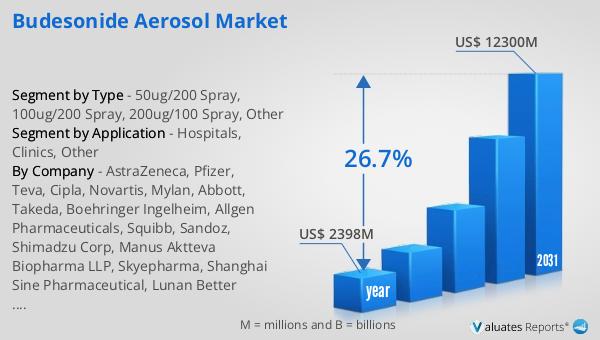
The global market for Budesonide Aerosol was valued at $2,398 million in 2024, with projections indicating a significant expansion to a revised size of $12,300 million by 2031. This growth represents a compound annual growth rate (CAGR) of 26.7% during the forecast period. Such a substantial increase underscores the rising demand for budesonide aerosol products, driven by factors such as the increasing prevalence of respiratory diseases, advancements in inhalation technology, and a growing awareness of the benefits of early and effective treatment. The market's robust growth trajectory reflects the critical role that budesonide aerosol plays in managing respiratory conditions like asthma and COPD, offering patients a reliable and effective treatment option. As healthcare systems worldwide continue to prioritize respiratory health, the demand for budesonide aerosol products is expected to rise, further fueling market expansion. The projected growth also highlights the importance of ongoing research and development efforts aimed at improving the efficacy and safety of budesonide formulations, as well as expanding their applications to a broader range of respiratory conditions. Overall, the Global Budesonide Aerosol Market is poised for significant growth, driven by a combination of clinical need, technological innovation, and increased awareness of respiratory health.
| Report Metric | Details |
| Report Name | Budesonide Aerosol Market |
| Accounted market size in year | US$ 2398 million |
| Forecasted market size in 2031 | US$ 12300 million |
| CAGR | 26.7% |
| Base Year | year |
| Forecasted years | 2025 - 2031 |
| Segment by Type |
|
| Segment by Application |
|
| Consumption by Region |
|
| By Company | AstraZeneca, Pfizer, Teva, Cipla, Novartis, Mylan, Abbott, Takeda, Boehringer Ingelheim, Allgen Pharmaceuticals, Squibb, Sandoz, Shimadzu Corp, Manus Aktteva Biopharma LLP, Skyepharma, Shanghai Sine Pharmaceutical, Lunan Better Pharmaceutical, Wellcome Australia Ltd, Synmosa Biopharma Corporation, Chiesi Farmaceutici S.p.A |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |