What is Global Clinical Research Organization Market?
The Global Clinical Research Organization Market is a vast and dynamic field that plays a crucial role in the healthcare industry. It refers to the outsourcing of pharmaceutical research services to third-party organizations known as Clinical Research Organizations (CROs). These organizations provide a wide range of services, including drug discovery, preclinical and clinical trial research, regulatory consulting, and commercialization services. The primary aim of these organizations is to help pharmaceutical and biotechnology companies in the development and testing of new drugs and medical devices. They ensure that these products are safe and effective for human use, adhering to stringent regulatory standards. The global market for these organizations is driven by the increasing demand for new and innovative medical treatments, the growing complexity of clinical trials, and the need for cost-effective drug development strategies.
Full-Service CRO, Functional Service Provision CRO in the Global Clinical Research Organization Market:
In the Global Clinical Research Organization Market, there are two main types of CROs: Full-Service CROs and Functional Service Provision (FSP) CROs. Full-Service CROs offer a comprehensive range of services, covering all stages of the drug development process. They provide end-to-end solutions, from drug discovery and preclinical research to clinical trials, regulatory approval, and post-marketing surveillance. On the other hand, FSP CROs specialize in specific functions or stages of the drug development process. They offer specialized services such as data management, biostatistics, medical writing, and site management. Both types of CROs play a vital role in the global market, helping pharmaceutical and biotech companies streamline their research and development activities, reduce costs, and accelerate time to market.
Pre-clinical, Clinical I-Ⅲ, Other in the Global Clinical Research Organization Market:
The Global Clinical Research Organization Market is utilized in various areas, including Pre-clinical, Clinical I-III, and Other areas. In the pre-clinical stage, CROs assist in the early stages of drug development, including in-vitro and in-vivo testing, toxicology studies, and formulation development. During the clinical stages I-III, CROs manage the design and execution of clinical trials, ensuring that they are conducted in compliance with Good Clinical Practice (GCP) guidelines. They also handle data management, statistical analysis, and report writing. In the 'Other' category, CROs provide services such as regulatory consulting, pharmacovigilance, and post-marketing surveillance. These services are critical in ensuring that drugs and medical devices are safe and effective, and meet the regulatory requirements of different countries.
Global Clinical Research Organization Market Outlook:
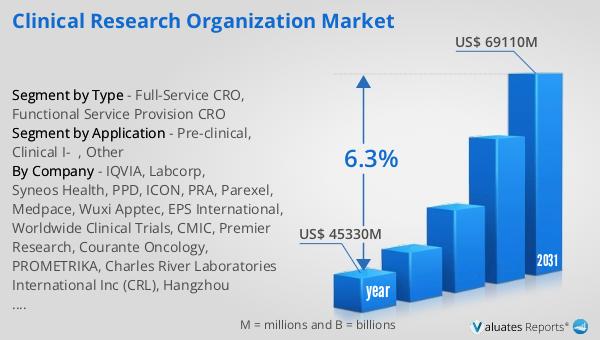
The future outlook for the Global Clinical Research Organization Market is promising. A new survey suggests that the market is set to grow significantly in the coming years. The global Clinical Research Organization market is expected to increase from its current value of US$ 41250 million in 2022 to an estimated US$ 61890 million by 2029. This represents a Compound Annual Growth Rate (CAGR) of 6.3% during the period from 2023 to 2029. This growth is driven by several factors, including the increasing demand for new drugs and medical treatments, the growing complexity of clinical trials, and the need for cost-effective drug development strategies. The role of CROs in the healthcare industry is becoming increasingly important, as they provide valuable support to pharmaceutical and biotech companies in their quest to develop safe and effective medical products.
| Report Metric | Details |
| Report Name | Clinical Research Organization Market |
| Accounted market size in 2022 | US$ 41250 million |
| Forecasted market size in 2029 | US$ 61890 million |
| CAGR | 6.3% |
| Base Year | 2022 |
| Forecasted years | 2023 - 2029 |
| Segment by Type |
|
| Segment by Application |
|
| By Region |
|
| By Company | IQVIA, Labcorp, Syneos Health, PPD, ICON, PRA, Parexel, Medpace, Wuxi Apptec, EPS International, Worldwide Clinical Trials, CMIC, Premier Research, Courante Oncology, PROMETRIKA, Charles River Laboratories International Inc (CRL), Hangzhou Tigermed, Clinipace, PSI CRO, Pierrel Research |
| Forecast units | USD million in value |
| Report coverage | Revenue and volume forecast, company share, competitive landscape, growth factors and trends |
